-
Name
3-Methylbutyl 3-methylbutanoate
- EINECS 211-536-1
- CAS No. 659-70-1
- Article Data46
- CAS DataBase
- Density 0.87 g/cm3
- Solubility 48.1mg/L at 20℃
- Melting Point -58.15°C
- Formula C10H20O2
- Boiling Point 194 °C at 760 mmHg
- Molecular Weight 172.268
- Flash Point 65.2 °C
- Transport Information
- Appearance colorless to yellowish transparent liquid
- Safety 26-36/37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Isovalericacid, isopentyl ester (6CI,7CI,8CI);Isopentyl alcohol, isovalerate (8CI);3-Methylbutyl 3-methylbutanoate;3-Methylbutyl 3-methylbutyrate;3-Methylbutylisovalerate;Isoamyl 3-methylbutanoate;NSC 6565;Solusterol;iso-Amyl isovalerate;
- PSA 26.30000
- LogP 2.62180
Synthetic route

| Conditions | Yield |
|---|---|
| With sodium bromate; hydrogen bromide In tetrachloromethane at 35 - 37℃; for 2h; | 96% |
| With Hoveyda-Grubbs catalyst second generation; potassium hydroxide; tricyclohexylphosphine In toluene at 110℃; for 12h; Reagent/catalyst; Schlenk technique; Inert atmosphere; | 90% |
| With pyridinium hydrobromide perbromide In water at 20℃; for 17h; | 87% |

| Conditions | Yield |
|---|---|
| With pyridine; dmap In dichloromethane at 0 - 20℃; for 2.5h; | 85.5% |

| Conditions | Yield |
|---|---|
| With sodium bromate; sodium hydrogensulfite for 2h; Ambient temperature; | A 5% B 76% |
| With iodosylbenzene; potassium bromide In water for 12h; sonication; | A 86 % Chromat. B n/a |

| Conditions | Yield |
|---|---|
| With pyridine; tetrachloromethane at 45℃; |

| Conditions | Yield |
|---|---|
| With ozone at 0℃; Reduzieren des vom entstandenen Wasserstoffperoxyd befreiten Reaktionsgemisches mit Zinkstaub in wenig Wasser; |
-
-
123-51-3
i-Amyl alcohol

-
A
-
503-74-2
3-methylbutyric acid

-
B
-
659-70-1
isopentyl 3-methylbutanoate

-
C
-
590-86-3
isovaleraldehyde

| Conditions | Yield |
|---|---|
| bei monatelangem Stehenlassen auch in Gegenwart von Katalysatoren; Gleichgewicht; pentyl alcohol of fermentation; |

-
-
503-74-2
3-methylbutyric acid

-
-
123-51-3
i-Amyl alcohol

-
-
590-86-3
isovaleraldehyde

-
-
659-70-1
isopentyl 3-methylbutanoate

| Conditions | Yield |
|---|---|
| Gleichgewicht im System bei monatelangem Stehenlassen; |


| Conditions | Yield |
|---|---|
| With copper uranium; hydrogen at 240 - 280℃; | |
| With Rh(PhBP3)(H)2(NCMe) In benzene-d6 at 20℃; for 0.0166667h; Tishchenko reaction; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| at 240℃; | |
| at 270℃; in Gegenwart von Wasserstoff erfolgt starke Zunahme der Esterbildung; |

-
-
25016-92-6
aluminium triisopentylate

-
-
590-86-3
isovaleraldehyde

-
A
-
123-51-3
i-Amyl alcohol

-
B
-
659-70-1
isopentyl 3-methylbutanoate

-
-
503-74-2
3-methylbutyric acid

-
-
123-51-3
i-Amyl alcohol

-
-
590-86-3
isovaleraldehyde

-
-
659-70-1
isopentyl 3-methylbutanoate

-
-
7726-95-6
bromine

-
-
110-46-3
isopentyl nitrite

-
A
-
659-70-1
isopentyl 3-methylbutanoate

-
B
-
107-82-4
i-pentyl bromide

| Conditions | Yield |
|---|---|
| anschliessenden Erwaermen; |


-
-
7664-93-9
sulfuric acid

-
-
7732-18-5
water

-
-
110-46-3
isopentyl nitrite

-
A
-
503-74-2
3-methylbutyric acid

-
B
-
659-70-1
isopentyl 3-methylbutanoate

| Conditions | Yield |
|---|---|
| at 100℃; |


-
-
659-70-1
isopentyl 3-methylbutanoate

| Conditions | Yield |
|---|---|
| With sulfuric acid at 38.5℃; bei der elektrolytischen Oxydation an einer Bleidioxyd-Anode; |
-
-
64-17-5
ethanol

-
-
123-51-3
i-Amyl alcohol

-
A
-
108-64-5
Ethyl isovalerate

-
B
-
123-92-2
3-methyl-1-butyl acetate

-
C
-
659-70-1
isopentyl 3-methylbutanoate

-
D
-
141-78-6
ethyl acetate

| Conditions | Yield |
|---|---|
| at 270℃; pentyl alcohol of fermentation; |


-
-
123-51-3
i-Amyl alcohol

-
A
-
503-74-2
3-methylbutyric acid

-
B
-
659-70-1
isopentyl 3-methylbutanoate

-
C
-
590-86-3
isovaleraldehyde

| Conditions | Yield |
|---|---|
| pentyl alcohol of fermentation; |

-
-
123-51-3
i-Amyl alcohol

-
A
-
503-74-2
3-methylbutyric acid

-
B
-
659-70-1
isopentyl 3-methylbutanoate

-
C
-
590-86-3
isovaleraldehyde

| Conditions | Yield |
|---|---|
| pentyl alcohol of fermentation; |

-
-
123-51-3
i-Amyl alcohol

-
A
-
503-74-2
3-methylbutyric acid

-
B
-
659-70-1
isopentyl 3-methylbutanoate

-
C
-
590-86-3
isovaleraldehyde

| Conditions | Yield |
|---|---|
| pentyl alcohol of fermentation; |

-
-
123-51-3
i-Amyl alcohol

-
A
-
503-74-2
3-methylbutyric acid

-
B
-
659-70-1
isopentyl 3-methylbutanoate

-
C
-
590-86-3
isovaleraldehyde

| Conditions | Yield |
|---|---|
| at 230℃; in je nach der Herstellung des Katalysators wechselden Mengen; | |
| at 330℃; in je nach der Herstellung des Katalysators wechselden Mengen; | |
| pentyl alcohol of fermentation; |

-
-
123-51-3
i-Amyl alcohol

-
-
7664-93-9
sulfuric acid

-
A
-
503-74-2
3-methylbutyric acid

-
B
-
659-70-1
isopentyl 3-methylbutanoate

-
C
-
590-86-3
isovaleraldehyde

-
-
1468-39-9
Isovaleric anhydride

-
A
-
503-74-2
3-methylbutyric acid

-
B
-
123-51-3
i-Amyl alcohol

-
C
-
659-70-1
isopentyl 3-methylbutanoate

-
D
-
590-86-3
isovaleraldehyde

| Conditions | Yield |
|---|---|
| at 180℃; |

-
-
544-01-4
isopentyl ether

-
-
10028-15-6
ozone

-
A
-
110-45-2
isopentyl formate

-
B
-
659-70-1
isopentyl 3-methylbutanoate

-
C
-
590-86-3
isovaleraldehyde

| Conditions | Yield |
|---|---|
| at 0℃; nach der Reduktion des Reaktionsprodukts mit Zinkstaub in Wasser; |

-
-
123-51-3
i-Amyl alcohol

-
A
-
503-74-2
3-methylbutyric acid

-
B
-
659-70-1
isopentyl 3-methylbutanoate

-
C
-
67-64-1
acetone

-
D
-
590-86-3
isovaleraldehyde

| Conditions | Yield |
|---|---|
| in schwach schefelsaurer Loesung; |


| Conditions | Yield |
|---|---|
| With copper-chromium oxide catalysts; hydrogen at 350 - 360℃; |

| Conditions | Yield |
|---|---|
| With copper | |
| With copper cerium | |
| With copper-zirconium catalysts |
-
-
10303-64-7
2-hydroxy-4-methylpentanoic acid

-
-
7664-93-9
sulfuric acid

-
A
-
503-74-2
3-methylbutyric acid

-
B
-
123-51-3
i-Amyl alcohol

-
C
-
659-70-1
isopentyl 3-methylbutanoate

| Conditions | Yield |
|---|---|
| at 38.5℃; elektrolytischen Oxydation an einer Bleidioxydanode; |


| Conditions | Yield |
|---|---|
| With C31H40MnN2O3P*C6H14O; potassium tert-butylate In toluene; 1,3,5-trimethyl-benzene at 110℃; for 48h; Schlenk technique; | 85% |

| Conditions | Yield |
|---|---|
| With phosphorus; bromine |
-
-
659-70-1
isopentyl 3-methylbutanoate

-
A
-
503-74-2
3-methylbutyric acid

-
B
-
117885-09-3
isopentyl-phenyl selenide

| Conditions | Yield |
|---|---|
| With Benzeneselenol; sodium hydride 1.) THF, 2.) HMPA, 12 h, reflux; Yield given. Multistep reaction. Yields of byproduct given; |
-
-
659-70-1
isopentyl 3-methylbutanoate

-
-
645-96-5
Benzeneselenol

-
A
-
503-74-2
3-methylbutyric acid

-
B
-
117885-09-3
isopentyl-phenyl selenide

| Conditions | Yield |
|---|---|
| With sodium hydride 1.) THF, 2.) HMPA, 12 h, reflux; Yield given. Multistep reaction. Yields of byproduct given; |

Isopentyl isopentanoate Chemical Properties
Detail of ISOVALERIC ACID, ISOPENTYL ESTER (659-70-1).
Molecular Structure: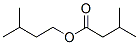
Name:ISOVALERIC ACID, ISOPENTYL ESTER
CAS Number:659-70-1
Molecular Formula:C10H20O2
Molecular Weight:172.30
EINECS:211-536-1
Density:0.854 g/mL at 25°C(lit.)
Boiling Point:192-193°C
Flash Point:152°F
Appearance:colorless to yellowish transparent liquid
FEMA:2085
refractive index:n20/D 1.412(lit.)
Merck:5121
NIST Chemistry Reference:659-70-1(NIST)
EPA Substance Registry System:659-70-1(EPA Substance)
Synonyms of ISOVALERIC ACID, ISOPENTYL ESTER (659-70-1):(3-methyl-1-butyl)3-methylbutanoate;3-methyl-butanoicaci3-methylbutylester;3-methyl-butanoicacid3-methyl-butylester;3-methylbutanoicacid3-methylbutylester;Apple oil;Appleoil;Butanoicacid,3-methyl-,3-methylbutylester;Isoamyl valerianate
Isopentyl isopentanoate Uses
ISOVALERIC ACID, ISOPENTYL ESTER (659-70-1) can be used for the preparation of food, daily flavor.And it is also be used as modifiers for the hawthorn flowers, apple flowers and other essences and used as the tobacco flavor.
Isopentyl isopentanoate Production
ISOVALERIC ACID, ISOPENTYL ESTER (659-70-1) has the production.
Use the separation of fusel oil obtained from isoamyl alcohol to isoamyl alcohol to refined through two oxidation derived from isovaleric formed in the presence of sulfuric acid esterification. By iso-amyl alcohol and isoamyl aldehyde vapor (240 ~ 280 ℃) in the presence of hydrogen through the copper-uranium catalysts derived column.
Isopentyl isopentanoate Toxicity Data With Reference
| 1. | skn-rbt 500 mg/24H MOD | FCTXAV Food and Cosmetics Toxicology. 16 (1978),789. | ||
| 2. | orl-rbt LD50:13,956 mg/kg | IMSUAI Industrial Medicine and Surgery. 41 (1972),31. |
Isopentyl isopentanoate Consensus Reports
Isopentyl isopentanoate Safety Profile
Safety Information of ISOVALERIC ACID, ISOPENTYL ESTER (659-70-1).
Hazard Codes:Xi
Risk Statements:36/37/38
Safety Statements:26-36/37/39
WGK Germany:2
RTECS:NY1508000
Related Products
- Isopentyl 4-methoxycinnamate
- Isopentyl ether
- Isopentyl formate
- Isopentyl hexanoate
- Isopentyl isobutyrate
- Isopentyl isopentanoate
- Isopentyl lactate
- Isopentyl nitrate
- Isopentyl phenethyl ether
- Isopentyl phenylacetate
- 65971-76-8
- 659720-02-2
- 659720-03-3
- 6597-22-4
- 659729-85-8
- 659730-32-2
- 659731-48-3
- 65973-52-6
- 65973-69-5
- 659737-57-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View