-
Name
SUCCINYL CHLORIDE
- EINECS 208-838-0
- CAS No. 543-20-4
- Article Data58
- CAS DataBase
- Density 1.389 g/cm3
- Solubility Reacts violently with water.
- Melting Point 16 °C
- Formula C4H4Cl2O2
- Boiling Point 193.4 °C at 760 mmHg
- Molecular Weight 154.981
- Flash Point 76.7 °C
- Transport Information UN 3265
- Appearance clear colourless to slightly brown liquid
- Safety 26-36/37/39-45
- Risk Codes 14-34
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms Succinylchloride (6CI,7CI,8CI);1,2-Bis(chlorocarbonyl)ethane;1,2-Ethanediylbis(chloroformate);NSC 87873;Succinic acid chloride;Succinicacid dichloride;Succinic chloride;Succinic dichloride;Succinoyl chloride;Succinoyl dichloride;Succinyl dichloride;
- PSA 34.14000
- LogP 1.29740
Synthetic route

| Conditions | Yield |
|---|---|
| With thionyl chloride for 4h; Inert atmosphere; Schlenk technique; Reflux; | 99% |
| With phosgene; N,N-dimethyl-formamide In toluene at 55℃; Product distribution / selectivity; | 98.2% |
| With oxalyl dichloride; N,N-dimethyl-formamide In dichloromethane at 0 - 20℃; for 3h; | 95% |

| Conditions | Yield |
|---|---|
| With thionyl chloride at 80℃; for 4h; | 93% |
| With thionyl chloride; zinc(II) chloride at 120 - 145℃; | |
| With thionyl chloride; copper(l) chloride; zinc(II) chloride at 120 - 145℃; |
-
-
108-30-5
succinic acid anhydride

-
-
4885-02-3
Dichloromethyl methyl ether

-
-
5435-44-9, 22243-66-9
(E)-3-Ureido-but-2-enoic acid ethyl ester

-
A
-
107-31-3
Methyl formate

-
B
-
543-20-4
succinoyl dichloride

-
-
108-30-5
succinic acid anhydride

-
-
4885-02-3
Dichloromethyl methyl ether

-
-
543-20-4
succinoyl dichloride

| Conditions | Yield |
|---|---|
| With zinc(II) chloride |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; nitromethane; chlorine |
-
-
108-30-5
succinic acid anhydride

-
-
10026-13-8, 874483-75-7
phosphorus pentachloride

-
-
543-20-4
succinoyl dichloride

| Conditions | Yield |
|---|---|
| at 120 - 130℃; |

-
-
543-20-4
succinoyl dichloride

| Conditions | Yield |
|---|---|
| With phosphorus pentachloride |

| Conditions | Yield |
|---|---|
| at 120 - 145℃; |

-
-
692-29-5
Succinic semialdehyde

-
-
10026-13-8, 874483-75-7
phosphorus pentachloride

-
A
-
543-20-4
succinoyl dichloride

-
-
110-94-1
1,5-pentanedioic acid

-
-
124-04-9
Adipic acid

-
-
110-15-6
succinic acid

-
A
-
2873-74-7
gloutaric dichloride

-
B
-
543-20-4
succinoyl dichloride

-
C
-
111-50-2
Adipic acid dichloride

| Conditions | Yield |
|---|---|
| With thionyl chloride at 20℃; |


| Conditions | Yield |
|---|---|
| With tetrabutyl-ammonium chloride; sodium hydroxide In dichloromethane; water at 0℃; for 0.0833333h; | 100% |
| With silica gel at 20℃; for 4h; | 72% |

| Conditions | Yield |
|---|---|
| In water at 0℃; for 0.5h; | 100% |
| In diethyl ether at -78 - 20℃; | 91% |
| In dichloromethane; water at 0 - 20℃; for 20h; Inert atmosphere; | 81% |
| With diethyl ether | |
| In water at 0℃; for 0.5h; |

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane at 20℃; for 72h; Inert atmosphere; | 100% |
| for 168h; Ambient temperature; | 63% |
-
-
188290-36-0
thiophene

-
-
543-20-4
succinoyl dichloride

-
-
13669-05-1
1,4-bis(thiophen-2-yl)butane-1,4-dione

| Conditions | Yield |
|---|---|
| With ethylaluminum dichloride In dichloromethane at 0℃; for 2h; Inert atmosphere; | 99% |
| With aluminum (III) chloride In N,N-dimethyl-formamide at 20℃; Inert atmosphere; | 84% |
| With aluminum (III) chloride In dichloromethane at 20℃; for 20h; Friedel-Crafts Acylation; | 82% |
-
-
67-56-1
methanol

-
-
563-43-9
ethylaluminum dichloride

-
-
543-20-4
succinoyl dichloride

-
-
2955-62-6
methyl 4-oxohexanoate

| Conditions | Yield |
|---|---|
| In dichloromethane at -40℃; for 3.5h; | 99% |
| Stage #1: ethylaluminum dichloride; succinoyl dichloride In dichloromethane at -40℃; for 3.5h; Stage #2: methanol In dichloromethane at -40℃; | 99% |


| Conditions | Yield |
|---|---|
| With sodium hydroxide for 0.05h; microwave irradiation; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: thymol; Amberlite IRA-400 (chloride form) resin With sodium hydroxide Stage #2: succinoyl dichloride In benzene | 98% |

-
-
501668-81-1
1,6-diamino-1,6-dideoxy-3,4-O-isopropylidene-D-mannitol

-
-
543-20-4
succinoyl dichloride

- poly(1,6-dideoxy-3,4-di-O-isopropylidene-D-mannitolsuccinimide); monomer(s): 1,6-diamino-1,6-dideoxy-3,4-di-O-isopropylidene-D-mannitol; succinoyl dichloride
-
poly(1,6-dideoxy-3,4-di-O-isopropylidene-D-mannitolsuccinimide); monomer(s): 1,6-diamino-1,6-dideoxy-3,4-di-O-isopropylidene-D-mannitol; succinoyl dichloride

| Conditions | Yield |
|---|---|
| With sodium carbonate In tetrachloromethane at 20℃; for 3h; | 98% |
-
-
741737-80-4
(Z)-2-(3,4-dimethoxy-phenyl)-3-(4-hydroxy-phenyl)-acrylonitrile

-
-
543-20-4
succinoyl dichloride

| Conditions | Yield |
|---|---|
| With pyridine | 98% |
-
-
14243-23-3
(μ-dithio)bis(tricarbonyliron)

-
-
543-20-4
succinoyl dichloride

-
-
4294-57-9
para-methylphenylmagnesium bromide

| Conditions | Yield |
|---|---|
| In tetrahydrofuran p-TolMgBr in THF was added under N2 with stirring to a THF soln. of Fe2-complex at -78°C, ClCOCH2CH2COCl (1/2 molar equiv) was added to the resulting anion soln. at -78°C, mixt. was allowed to warm to room temp.; volatiles were removed, residue purified by column chromy.; elem. anal.; | 98% |

-
-
56777-24-3
benzyl (S)-Lactate

-
-
543-20-4
succinoyl dichloride

-
-
314069-31-3
Succinic acid bis-((S)-1-benzyloxycarbonyl-ethyl) ester

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 25℃; under 2585.74 Torr; for 16h; | 97% |
| With pyridine In dichloromethane at 0℃; |

| Conditions | Yield |
|---|---|
| With dmap In dichloromethane at 20℃; for 0.216667h; | 97% |
| With dmap In dichloromethane at 20℃; for 0.133333h; Inert atmosphere; | 97% |
-
-
543-20-4
succinoyl dichloride

-
-
159146-73-3
12-(p-aminophenyl)-9,9-dimethyl-8,9,10,11-tetrahydrobenzacridin-11-one

| Conditions | Yield |
|---|---|
| In acetone at 5℃; for 0.25h; | 96% |

| Conditions | Yield |
|---|---|
| In pyridine at 20℃; for 24h; | 95% |
| With benzene |
-
-
3025-88-5
2,5-dimethyl-2,5-dihydroperoxyhexane

-
-
543-20-4
succinoyl dichloride

-
-
88-95-9
Phthaloyl dichloride

-
-
78491-83-5
2-(4-{2-[4-(3-{4-[3-(4-Hydroperoxy-1,1,4-trimethyl-pentylperoxycarbonyl)-propionylperoxy]-1,1,4-trimethyl-pentylperoxycarbonyl}-propionylperoxy)-1,1,4-trimethyl-pentylperoxycarbonyl]-benzoylperoxy}-1,1,4-trimethyl-pentylperoxycarbonyl)-benzoic acid

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 20℃; for 1.5h; | 95% |

-
-
543-20-4
succinoyl dichloride


| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide for 3h; Ambient temperature; | 95% |
-
-
90-33-5, 79566-13-5
7-hydroxy-4-methyl-chromen-2-one

-
-
543-20-4
succinoyl dichloride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 2h; | 95% |
-
-
543-20-4
succinoyl dichloride

-
-
292140-08-0
Nε-lauroyl-L-lysine ethyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 20℃; for 24h; | 95% |

| Conditions | Yield |
|---|---|
| With tetrabutyl-ammonium chloride; sodium hydroxide In dichloromethane; water at 0℃; for 0.0833333h; | 95% |

| Conditions | Yield |
|---|---|
| With antimonypentachloride In tetrachloromethane for 5h; Heating; | 94% |
| With antimonypentachloride In dichloromethane 1) -40 to 25 deg C, 2) 25 deg C, 3 h; | 75% |
-
-
543-20-4
succinoyl dichloride

-
-
117358-75-5
7-(p-Aminophenyl)-10,10-dimethyl-8,9,10,11-tetrahydrobenz[c]acridin-8-one

| Conditions | Yield |
|---|---|
| In acetone at 5℃; for 0.25h; | 94% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; Amberlite IRA-400 chloride form In water; acetone | 94% |
| Stage #1: 2-Mercaptobenzothiazole With aluminum oxide; silica gel; sodium carbonate; fly ash microwave oven; Stage #2: succinoyl dichloride microwave irradiation; |

| Conditions | Yield |
|---|---|
| Stage #1: N-hydroxyphthalimide With sodium hydroxide; Amberlite IRA-400 chloride form Stage #2: succinoyl dichloride In tetrahydrofuran at 20℃; | 94% |
| With pyridine at 20℃; for 24h; | 60% |

| Conditions | Yield |
|---|---|
| In pyridine at 20℃; for 24h; | 93% |
-
-
1020108-12-6
(1R,2R,3S,5S)-3-(3,4-dichlorophenyl)-2-hydroxymethyl-8-thiabicyclo[3.2.1]octane

-
-
543-20-4
succinoyl dichloride

-
-
1020108-07-9
(3β-(3,4-dichlorophenyl)-8-thiabicyclo[3.2.1]octan-2β-yl)methyl,(3β-(3,4-dichlorophenyl)-8-thiabicyclo[3.2.1]octan-2β-yl)methyl succinate

| Conditions | Yield |
|---|---|
| With dmap In dichloromethane at 0 - 20℃; | 93% |

| Conditions | Yield |
|---|---|
| Stage #1: octaethylene glycol monomethyl ether With sodium hydride In tetrahydrofuran at -20℃; for 0.5h; Stage #2: succinoyl dichloride In tetrahydrofuran at -20 - 20℃; | 93% |

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride In benzene at 0 - 20℃; Friedel-Crafts acylation; Inert atmosphere; | 93% |

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride In benzene at 0 - 20℃; Friedel-Crafts acylation; Inert atmosphere; | 92% |
| Stage #1: succinoyl dichloride; toluene With aluminum (III) chloride In dichloromethane at 0℃; for 0.5h; Stage #2: With hydrogenchloride | 73% |
| With aluminium trichloride | |
| With aluminium trichloride |
Succinyl chloride Consensus Reports
Reported in EPA TSCA Inventory.
Succinyl chloride Specification
The Succinyl chloride with CAS registry number of 543-20-4 is also called 1,2-Ethanediylbis(chloroformate). The IUPAC name is 543-20-4. Its EINECS registry number is 208-838-0. In addition, the formula is C4H4Cl2O2 and the molecular weight is 154.97936. It is a kind of clear colourless to slightly brown liquid. It is sensitive to moisture. What's more, it is the intermediate of synthetic resins, paints and succinylcholine chloride.
Physical properties about this chemical are: (1)ACD/LogP: 1.01; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.01; (4)ACD/LogD (pH 7.4): 1.01; (5)ACD/BCF (pH 5.5): 3.42; (6)ACD/BCF (pH 7.4): 3.42; (7)ACD/KOC (pH 5.5): 84; (8)ACD/KOC (pH 7.4): 84; (9)#H bond acceptors: 2; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 3; (12)Polar Surface Area: 34.14 Å2; (13)Index of Refraction: 1.456; (14)Molar Refractivity: 30.33 cm3; (15)Molar Volume: 111.5 cm3; (16)Polarizability: 12.02 ×10-24cm3; (17)Surface Tension: 38.7 dyne/cm; (18)Density: 1.389 g/cm3; (19)Flash Point: 76.7 °C; (20)Enthalpy of Vaporization: 42.96 kJ/mol; (21)Boiling Point: 193.4 °C at 760 mmHg; (22)Vapour Pressure: 0.466 mmHg at 25°C.
Preparation Succinyl chloride: it can be prepared by butanedioic acid and phosphorus pentylchloride. You can put butanedioic acid, phosphorus pentylchloride and chloroform into reactor as sequence. When the reaction turned slow, you should heat the mixture to refluxing. when the reaction products become liquid, steam the low viscosity and recover chloroform. The yield is about 88%.
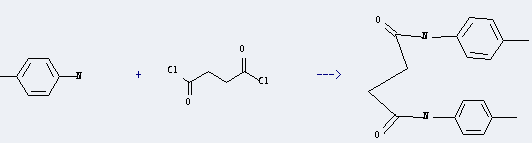
Uses of Succinyl chloride: it can react with 4-methyl-aniline to get N,N'-di-p-tolyl-succinamide. This reaction will need reagent pyridine. The reaction time is 24 hours at reaction temperature of 20 °C. The yield is about 91%.
When you are using this chemical, please be cautious about it as the following:
It can react violently with water and cause burns. You should wear suitable protective clothing, gloves and eye/face protection when use it. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. And in case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.).
You can still convert the following datas into molecular structure:
(1)SMILES: ClC(=O)CCC(Cl)=O
(2)InChI: InChI=1/C4H4Cl2O2/c5-3(7)1-2-4(6)8/h1-2H2
(3)InChIKey: IRXBNHGNHKNOJI-UHFFFAOYAK
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LDLo | intraperitoneal | 62500ug/kg (62.5mg/kg) | "Summary Tables of Biological Tests," National Research Council Chemical-Biological Coordination Center. Vol. 4, Pg. 111, 1952. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View