-
Name
Tanshinone IIA
- EINECS
- CAS No. 568-72-9
- Article Data9
- CAS DataBase
- Density 1.209 g/cm3
- Solubility Soluble in Alcohol and DMSO
- Melting Point 205-207 °C
- Formula C19H18O3
- Boiling Point 480.7 °C at 760 mmHg
- Molecular Weight 294.35
- Flash Point 236.4 °C
- Transport Information
- Appearance Cherry crystal
- Safety 24/25
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Phenanthro[1,2-b]furan-10,11-dione,6,7,8,9-tetrahydro-1,6,6-trimethyl-;TanshinoneIIA (7CI);1,6,6-Trimethyl-6,7,8,9-tetrahydrophenanthro[1,2-b]furan-10,11-dione;Dan Shenketone;NSC 686518;NSC 686519;Tanshinone B;Tanshinone II;
- PSA 47.28000
- LogP 4.24790
Synthetic route
-
-
17545-07-2, 35825-57-1
(+/-)-cryptotanshinone

-
-
568-72-9
tanshinone IIA

| Conditions | Yield |
|---|---|
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In benzene at 20℃; | 95% |
-
-
35825-57-1
Cryptotanshinone

-
-
568-72-9
tanshinone IIA

| Conditions | Yield |
|---|---|
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In benzene at 25℃; for 42h; | 91% |

-
-
568-72-9
tanshinone IIA

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen In tetrahydrofuran | 40% |

-
-
568-72-9
tanshinone IIA

| Conditions | Yield |
|---|---|
| With sodium In tetrahydrofuran at 50℃; for 1h; Inert atmosphere; | 40% |
-
-
18238-29-4
6,6-dimethyl-1-vinylcyclohex-1-ene

-
-
113297-21-5
4,5-dihydro-3-methylbenzo[1,2-b]furan-4,5-dione

-
A
-
568-72-9
tanshinone IIA

-
B
-
118950-00-8
3,8,8-Trimethyl-8,9,10,11-tetrahydro-phenanthro[4,3-b]furan-4,5-dione

| Conditions | Yield |
|---|---|
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone 1) neat, ultrasound, 45 deg C, 2h, (or benzene, 12h, reflux), 2) benzene, 12h, reflux; Yield given. Multistep reaction. Yields of byproduct given; | |
| Yield given. Multistep reaction. Yields of byproduct given; | |
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone 1) benzene, reflux, 12h, (or ultrasonification, 45 deg C, 2h), 2) benzene, 12h, reflux; Yield given. Multistep reaction. Yields of byproduct given; |


| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1: 1.) n-BuLi, TMEDA / 1.) hexane, 45 deg C, 30 min, 2.) THF, 55-60 deg C, 20 h 2: 97 percent / AlCl3 / CH2Cl2 / 0.25 h / 0 °C 3: 95 percent / BBr3 / CH2Cl2 / 4 h / -78 - 25 °C 4: 87 percent / DMAP / pyridine / 1.) 0 deg C -> 25 deg C, 1 h, 2.) 25 deg C, 48 h 5: 71 percent / LiCl, 4A sieves, Me4Sn, 2,6-di-tert-butylhydroxytoluene / PdCl2(dppf) / dimethylformamide / 12 h / 90 °C / 2585.7 Torr 6: 1.) LiHMDS, 2,2,2-trifluoroethyl trifluoroacetate, 2.) MsN3, Et3N / 1.) THF, -78 deg C, 45 min, 2.) MeCN, H2O, 25 deg C, 6 h 7: 64 percent / benzene / 24 h / Ambient temperature; Irradiation 8: 84 percent / n-Bu4NF, O2 / tetrahydrofuran / 40 h / 25 °C 9: 100 percent / conc. H2SO4 / ethanol / 0.75 h / 25 °C 10: 91 percent / DDQ / benzene / 42 h / 25 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1: 93 percent / PdCl2(dppf) / tetrahydrofuran / 15 h / Heating 2: 97 percent / AlCl3 / CH2Cl2 / 0.25 h / 0 °C 3: 95 percent / BBr3 / CH2Cl2 / 4 h / -78 - 25 °C 4: 87 percent / DMAP / pyridine / 1.) 0 deg C -> 25 deg C, 1 h, 2.) 25 deg C, 48 h 5: 71 percent / LiCl, 4A sieves, Me4Sn, 2,6-di-tert-butylhydroxytoluene / PdCl2(dppf) / dimethylformamide / 12 h / 90 °C / 2585.7 Torr 6: 1.) LiHMDS, 2,2,2-trifluoroethyl trifluoroacetate, 2.) MsN3, Et3N / 1.) THF, -78 deg C, 45 min, 2.) MeCN, H2O, 25 deg C, 6 h 7: 64 percent / benzene / 24 h / Ambient temperature; Irradiation 8: 84 percent / n-Bu4NF, O2 / tetrahydrofuran / 40 h / 25 °C 9: 100 percent / conc. H2SO4 / ethanol / 0.75 h / 25 °C 10: 91 percent / DDQ / benzene / 42 h / 25 °C View Scheme |
-
-
171979-69-4
5,5-dimethyl-5,6,7,8-tetrahydronaphthalen-1-ol

-
-
568-72-9
tanshinone IIA

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 87 percent / DMAP / pyridine / 1.) 0 deg C -> 25 deg C, 1 h, 2.) 25 deg C, 48 h 2: 71 percent / LiCl, 4A sieves, Me4Sn, 2,6-di-tert-butylhydroxytoluene / PdCl2(dppf) / dimethylformamide / 12 h / 90 °C / 2585.7 Torr 3: 1.) LiHMDS, 2,2,2-trifluoroethyl trifluoroacetate, 2.) MsN3, Et3N / 1.) THF, -78 deg C, 45 min, 2.) MeCN, H2O, 25 deg C, 6 h 4: 64 percent / benzene / 24 h / Ambient temperature; Irradiation 5: 84 percent / n-Bu4NF, O2 / tetrahydrofuran / 40 h / 25 °C 6: 100 percent / conc. H2SO4 / ethanol / 0.75 h / 25 °C 7: 91 percent / DDQ / benzene / 42 h / 25 °C View Scheme |
-
-
33214-70-9
1,1-dimethyl-5-methoxytetralin

-
-
568-72-9
tanshinone IIA

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: 95 percent / BBr3 / CH2Cl2 / 4 h / -78 - 25 °C 2: 87 percent / DMAP / pyridine / 1.) 0 deg C -> 25 deg C, 1 h, 2.) 25 deg C, 48 h 3: 71 percent / LiCl, 4A sieves, Me4Sn, 2,6-di-tert-butylhydroxytoluene / PdCl2(dppf) / dimethylformamide / 12 h / 90 °C / 2585.7 Torr 4: 1.) LiHMDS, 2,2,2-trifluoroethyl trifluoroacetate, 2.) MsN3, Et3N / 1.) THF, -78 deg C, 45 min, 2.) MeCN, H2O, 25 deg C, 6 h 5: 64 percent / benzene / 24 h / Ambient temperature; Irradiation 6: 84 percent / n-Bu4NF, O2 / tetrahydrofuran / 40 h / 25 °C 7: 100 percent / conc. H2SO4 / ethanol / 0.75 h / 25 °C 8: 91 percent / DDQ / benzene / 42 h / 25 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1: 1.) n-BuLi, TMEDA / 1.) hexane, 45 deg C, 30 min, 2.) THF, 55-60 deg C, 20 h 2: 97 percent / AlCl3 / CH2Cl2 / 0.25 h / 0 °C 3: 95 percent / BBr3 / CH2Cl2 / 4 h / -78 - 25 °C 4: 87 percent / DMAP / pyridine / 1.) 0 deg C -> 25 deg C, 1 h, 2.) 25 deg C, 48 h 5: 71 percent / LiCl, 4A sieves, Me4Sn, 2,6-di-tert-butylhydroxytoluene / PdCl2(dppf) / dimethylformamide / 12 h / 90 °C / 2585.7 Torr 6: 1.) LiHMDS, 2,2,2-trifluoroethyl trifluoroacetate, 2.) MsN3, Et3N / 1.) THF, -78 deg C, 45 min, 2.) MeCN, H2O, 25 deg C, 6 h 7: 64 percent / benzene / 24 h / Ambient temperature; Irradiation 8: 84 percent / n-Bu4NF, O2 / tetrahydrofuran / 40 h / 25 °C 9: 100 percent / conc. H2SO4 / ethanol / 0.75 h / 25 °C 10: 91 percent / DDQ / benzene / 42 h / 25 °C View Scheme |

-
-
568-72-9
tanshinone IIA

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1: 93 percent / PdCl2(dppf) / tetrahydrofuran / 15 h / Heating 2: 97 percent / AlCl3 / CH2Cl2 / 0.25 h / 0 °C 3: 95 percent / BBr3 / CH2Cl2 / 4 h / -78 - 25 °C 4: 87 percent / DMAP / pyridine / 1.) 0 deg C -> 25 deg C, 1 h, 2.) 25 deg C, 48 h 5: 71 percent / LiCl, 4A sieves, Me4Sn, 2,6-di-tert-butylhydroxytoluene / PdCl2(dppf) / dimethylformamide / 12 h / 90 °C / 2585.7 Torr 6: 1.) LiHMDS, 2,2,2-trifluoroethyl trifluoroacetate, 2.) MsN3, Et3N / 1.) THF, -78 deg C, 45 min, 2.) MeCN, H2O, 25 deg C, 6 h 7: 64 percent / benzene / 24 h / Ambient temperature; Irradiation 8: 84 percent / n-Bu4NF, O2 / tetrahydrofuran / 40 h / 25 °C 9: 100 percent / conc. H2SO4 / ethanol / 0.75 h / 25 °C 10: 91 percent / DDQ / benzene / 42 h / 25 °C View Scheme |
-
-
109664-02-0
5,6,7,8-tetrahydro-3-hydroxy-2-[(1R)-2-hydroxy-1-methylethyl]-8,8-dimethyl-1,4-phenanthrenedione

-
-
568-72-9
tanshinone IIA

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 100 percent / conc. H2SO4 / ethanol / 0.75 h / 25 °C 2: 91 percent / DDQ / benzene / 42 h / 25 °C View Scheme |
-
-
171979-71-8
1,1-dimethyl-5-tetralyl trifluoromethanesulfonate

-
-
568-72-9
tanshinone IIA

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 71 percent / LiCl, 4A sieves, Me4Sn, 2,6-di-tert-butylhydroxytoluene / PdCl2(dppf) / dimethylformamide / 12 h / 90 °C / 2585.7 Torr 2: 1.) LiHMDS, 2,2,2-trifluoroethyl trifluoroacetate, 2.) MsN3, Et3N / 1.) THF, -78 deg C, 45 min, 2.) MeCN, H2O, 25 deg C, 6 h 3: 64 percent / benzene / 24 h / Ambient temperature; Irradiation 4: 84 percent / n-Bu4NF, O2 / tetrahydrofuran / 40 h / 25 °C 5: 100 percent / conc. H2SO4 / ethanol / 0.75 h / 25 °C 6: 91 percent / DDQ / benzene / 42 h / 25 °C View Scheme |
-
-
171979-72-9
3-((S)-1-((tert-butyldimethylsilyl)oxy)-2-propyl)-7,7-dimethyl-4-hydroxy-2-((triisopropylsilyl)oxy)-7,8,9,10-tetrahydrophenanthrene

-
-
568-72-9
tanshinone IIA

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 84 percent / n-Bu4NF, O2 / tetrahydrofuran / 40 h / 25 °C 2: 100 percent / conc. H2SO4 / ethanol / 0.75 h / 25 °C 3: 91 percent / DDQ / benzene / 42 h / 25 °C View Scheme |
-
-
568-72-9
tanshinone IIA

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

| Conditions | Yield |
|---|---|
| With trichlorophosphate at 20℃; for 2h; | 100% |
| With trichlorophosphate at 20℃; for 2.5h; Vilsmeier-Haack Formylation; | 97.3% |
| With trichlorophosphate at 70 - 80℃; for 2h; | 67% |
-
-
568-72-9
tanshinone IIA

| Conditions | Yield |
|---|---|
| With potassium iodate; acetic acid; potassium iodide In water at 80℃; for 1h; | 98.99% |
| With N-iodo-succinimide; acetic acid In dichloromethane at -40℃; for 6h; |
-
-
568-72-9
tanshinone IIA

| Conditions | Yield |
|---|---|
| With sodium hydrogen sulfate; acetic anhydride; 1,4,7,10-tetraoxa-15-azacyclopentadecane at 20℃; for 1h; Reagent/catalyst; Temperature; Green chemistry; | 96% |
| Stage #1: tanshinone-IIA With sulfur trioxide-N,N-dimethylformamide complex In N,N-dimethyl-formamide at 100℃; Stage #2: With sodium hydroxide In ethanol; water pH=7.5 - 9; Reagent/catalyst; Solvent; Temperature; | 93% |
| Stage #1: tanshinone-IIA With sulfuric acid; acetic anhydride; acetic acid In dichloromethane at 25℃; for 0.0666667h; Stage #2: With sodium chloride In dichloromethane; water; Petroleum ether for 0.0833333h; Temperature; | 72.6% |
| Stage #1: tanshinone-IIA With sulfuric acid; acetic anhydride; acetic acid at 25℃; Stage #2: With sodium chloride In water Product distribution / selectivity; | |
| Stage #1: tanshinone-IIA With sulfuric acid; acetic anhydride; acetic acid In dichloromethane Flow reactor; Heating; Stage #2: With sodium chloride |
-
-
568-72-9
tanshinone IIA

-
-
65-85-0
benzoic acid

-
-
1392502-49-6
1,6,6-trimethyl-10,11-dioxo-6,7,8,9,10,11-hexahydrophenanthro[1,2-b]furan-9-yl benzoate

| Conditions | Yield |
|---|---|
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical In chlorobenzene at 120℃; for 24h; Sealed tube; regioselective reaction; | 95% |
-
-
568-72-9
tanshinone IIA

-
-
333-27-7
methyl trifluoromethanesulfonate

-
-
120727-43-7
10,11-Dimethoxy-1,6,6-trimethyl-6,7,8,9-tetrahydro-phenanthro[1,2-b]furan

| Conditions | Yield |
|---|---|
| Stage #1: tanshinone-IIA In tetrahydrofuran at -30℃; for 0.0833333h; Stage #2: methyl trifluoromethanesulfonate In tetrahydrofuran for 0.5h; | 95% |

| Conditions | Yield |
|---|---|
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical In chlorobenzene at 120℃; for 24h; Sealed tube; regioselective reaction; | 92% |

| Conditions | Yield |
|---|---|
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical In chlorobenzene at 120℃; for 24h; Sealed tube; regioselective reaction; | 92% |
-
-
568-72-9
tanshinone IIA

-
-
802294-64-0
propionic acid

| Conditions | Yield |
|---|---|
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical In chlorobenzene at 120℃; for 24h; Reagent/catalyst; Solvent; Sealed tube; regioselective reaction; | 91% |

| Conditions | Yield |
|---|---|
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical In chlorobenzene at 120℃; for 24h; Sealed tube; regioselective reaction; | 90% |
-
-
568-72-9
tanshinone IIA

-
-
579-75-9
2-Methoxybenzoic acid

-
-
1392502-51-0
1,6,6-trimethyl-10,11-dioxo-6,7,8,9,10,11-hexahydrophenanthro[1,2-b]furan-9-yl 2-methoxybenzoate

| Conditions | Yield |
|---|---|
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical In chlorobenzene at 120℃; for 24h; Sealed tube; regioselective reaction; | 90% |
-
-
568-72-9
tanshinone IIA

-
-
108-24-7
acetic anhydride

-
-
98796-69-1
10,11-Diacetoxy-1,6,6-trimethyl-6,7,8,9-tetrahydro-phenanthro[1,2-b]furan

| Conditions | Yield |
|---|---|
| Stage #1: tanshinone-IIA With palladium 10% on activated carbon; hydrogen In tetrahydrofuran at 15 - 25℃; Stage #2: acetic anhydride With pyridine In tetrahydrofuran at 15 - 25℃; for 3h; | 90% |
-
-
50-00-0
formaldehyd

-
-
1151904-84-5
4,5,6,7-tetrahydrothieno[3,2-c]pyridin-2-yl acetate hydrochloride

-
-
568-72-9
tanshinone IIA

| Conditions | Yield |
|---|---|
| With zinc diacetate In dimethyl sulfoxide at 60℃; for 24h; | 89% |


| Conditions | Yield |
|---|---|
| With zinc diacetate; acetic acid In chloroform Reflux; Inert atmosphere; | 89% |


| Conditions | Yield |
|---|---|
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical In chlorobenzene at 120℃; for 24h; Sealed tube; regioselective reaction; | 88% |
-
-
568-72-9
tanshinone IIA

-
-
61077-78-9
6,7,8,9-tetrahydro-1,6,6-trimethylfuronaphth<2,1-e>oxepine-10,12-dione

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen; acetic acid In water; benzene at 25℃; for 8h; | 86% |
| With sodium hydrogencarbonate; 3-chloro-benzenecarboperoxoic acid In dichloromethane at 20℃; for 1h; | 21% |

| Conditions | Yield |
|---|---|
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical at 120℃; for 24h; Sealed tube; regioselective reaction; | 85% |

| Conditions | Yield |
|---|---|
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical In toluene at 120℃; for 48h; Sealed tube; | 85% |
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical In toluene at 120℃; for 48h; | 85% |

| Conditions | Yield |
|---|---|
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical In chlorobenzene at 120℃; for 24h; Sealed tube; regioselective reaction; | 81% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In acetonitrile at 50℃; for 72h; | 81% |

| Conditions | Yield |
|---|---|
| With dmap; iron In tetrahydrofuran for 6h; Reflux; Inert atmosphere; | 81% |
-
-
568-72-9
tanshinone IIA

-
-
120727-39-1
2-bromo-10,11-dioxo-1,6,6-trimethyl-6,7,8,9,10,11-hexahydro-phenanthro[1,2-b]furan

| Conditions | Yield |
|---|---|
| With bromine; acetic acid at 20℃; for 4h; | 80% |
| With bromine In tetrachloromethane at 20℃; for 0.333333h; | |
| With N-Bromosuccinimide; acetic acid at 20℃; for 6h; | |
| With hydrogen bromide; bromine In dichloromethane at 20℃; for 0.5h; Darkness; |

| Conditions | Yield |
|---|---|
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical In chlorobenzene at 120℃; for 24h; Sealed tube; regioselective reaction; | 80% |

| Conditions | Yield |
|---|---|
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical In chlorobenzene at 120℃; for 24h; Sealed tube; regioselective reaction; | 80% |

| Conditions | Yield |
|---|---|
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical In toluene at 120℃; for 48h; | 80% |

| Conditions | Yield |
|---|---|
| With zirconium(IV) chloride In dichloromethane Reflux; | 79% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In acetonitrile at 50℃; for 72h; | 79% |

| Conditions | Yield |
|---|---|
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical In chlorobenzene at 120℃; for 36h; Reagent/catalyst; Solvent; Sealed tube; regioselective reaction; | 78% |
-
-
568-72-9
tanshinone IIA

| Conditions | Yield |
|---|---|
| With Selectfluor In water; acetonitrile at 80℃; for 12h; Reagent/catalyst; Solvent; Sealed tube; | 77% |
| With Selectfluor In water; acetonitrile at 80℃; for 12h; | 77% |
Tanshinone IIA Chemical Properties
Molecular Formula: C14H10O3
Molar mass: 226.23 g/mol
Density: 1.209 g/cm3
Flash Point: 236.4 °C
Index of Refraction: 1.587
Boiling Point: 480.7 °C at 760 mmHg
Vapour Pressure: 2.12E-09 mmHg at 25 °C
Product categories of Tanshinone IIA (CAS NO.568-72-9): Plant Oils, Toxins, Phenolic Acids & Derivatives;Danshen
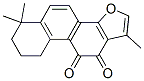
Structure of Tanshinone IIA (CAS NO.568-72-9):
XLogP3-AA: 4.3
H-Bond Donor: 0
H-Bond Acceptor: 3
IUPAC Name of Tanshinone IIA (CAS NO.568-72-9): 1,6,6-Trimethyl-8,9-dihydro-7H-naphtho[1,2-g][1]benzofuran-10,11-dione
Canonical SMILES: CC1=COC2=C1C(=O)C(=O)C3=C2C=CC4=C3CCCC4(C)C
InChI: InChI=1S/C19H18O3/c1-10-9-22-18-12-6-7-13-11(5-4-8-19(13,
2)3)15(12)17(21)16(20)14(10)18/h6-7,9H,4-5,8H2,1-3H3
InChIKey: HYXITZLLTYIPOF-UHFFFAOYSA-N
Tanshinone IIA Specification
Tanshinone IIA , its cas register number is 568-72-9. It also can be called Phenanthro[1,2-b]furan-10,11-dione, 6,7,8,9-tetrahydro-1,6,6-trimethyl- and 1,6,6-Trimethyl-6,7,8,9-tetrahydrophenanthro[1,2-b]furan-10,11-dione .
Related Products
- Tanshinone I
- Tanshinone IIA
- 568-73-0
- 5687-48-9
- 56875-23-1
- 56877-43-1
- 5687-92-3
- 56879-47-1
- 56881-31-3
- 56881-90-4
- 56-88-2
- 56883-39-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View