-
Name
tert-Butyl carbamate
- EINECS 224-209-3
- CAS No. 4248-19-5
- Article Data72
- CAS DataBase
- Density 0.99 g/cm3
- Solubility Soluble in methylene chloride, chloroform, and alcohols. Slightly soluble in petroleum ether and water.
- Melting Point 105-108 °C(lit.)
- Formula C5H11NO2
- Boiling Point 195.979 °C at 760 mmHg
- Molecular Weight 117.148
- Flash Point 82.407 °C
- Transport Information
- Appearance White to slightly yellow needles
- Safety 39-26
- Risk Codes 36
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Carbamicacid, tert-butyl ester (6CI,7CI,8CI);Carbamic acid tert-butyl ester;NSC131089;O-tert-Butyl carbamate;N-T-Butoxycarbonyl-Amide;
- PSA 52.32000
- LogP 1.58050
Synthetic route
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
4248-19-5
tert-butyl carbamate

| Conditions | Yield |
|---|---|
| With ammonia In ethanol at 0 - 20℃; | 98% |
| With ammonium hydroxide In ethanol; water at -10 - 20℃; for 18.5833h; | 95% |
| With ammonia In methanol at 0 - 20℃; for 7h; | 94% |

| Conditions | Yield |
|---|---|
| With merrifield anchored iron(II)-anthra catalyst In 1,4-dioxane at 120℃; for 6.5h; | 81% |
| With cholin chloride ZnCl2; iron oxide at 130℃; for 6h; Green chemistry; | 69% |
-
-
70678-13-6
1,2-bis(1-isocyanato-1-methylethyl)diazene

-
-
75-65-0
tert-butyl alcohol

-
A
-
4248-19-5
tert-butyl carbamate

-
B
-
112700-85-3
5,5-dimethyl-Δ1-1,2,4-triazolin-3-one

| Conditions | Yield |
|---|---|
| for 22h; Heating; | A 29% B 74% |


| Conditions | Yield |
|---|---|
| With perchloric acid on silica gel at 20℃; for 0.75h; | 74% |
| With trifluoroacetic acid 2.) CH2Cl2; Multistep reaction; |
-
-
1330763-30-8
(4-aminotetrahydrofuran-3-yl)carbamic acid tert-butyl ester

-
-
335276-54-5
4-[(4-fluorophenyl)methyl]-cyclohexanone

-
A
-
335388-53-9
tert-butyl (3S,4S)-4-{[4-(4-fluorobenzyl)cyclohexyl]amino}tetrahydro-3-furanylcarbamate

-
B
-
4248-19-5
tert-butyl carbamate

| Conditions | Yield |
|---|---|
| With sodium tris(acetoxy)borohydride In methanol; dichloromethane; 1,2-dichloro-ethane | A 53% B n/a |


| Conditions | Yield |
|---|---|
| With para-dodecylbenzenesulfonic acid In neat (no solvent) at 60℃; for 0.5h; Green chemistry; | 52% |
| With DBSA at 60℃; for 1h; | |
| With DBSA at 60℃; for 1h; | |
| With DBSA at 60℃; for 1h; |
-
-
22288-78-4
Methyl 3-aminothiophene-2-carboxylate

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
4248-19-5
tert-butyl carbamate

| Conditions | Yield |
|---|---|
| With dmap In hexane; dichloromethane; ethyl acetate | 36% |
-
-
85535-53-1
Boc-Lys-NH2 hemioxalate

-
A
-
4248-19-5
tert-butyl carbamate

-
B
-
373-04-6
6-aminocaproic amide

-
C
-
85535-54-2
tert-butyl (S)-(6-amino-1-hydroxyhexan-2-yl)carbamate

| Conditions | Yield |
|---|---|
| With ammonia; sodium | A 14.7% B 22% C 20 % Chromat. |
| With ammonia; sodium Product distribution; | A 14.7% B 22% C 20 % Chromat. |

-
-
83351-75-1
tert-butyl (S)-(1-amino-3-(4-hydroxyphenyl)-1-oxopropan-2-yl)carbamate

-
A
-
4248-19-5
tert-butyl carbamate

-
B
-
23838-70-2
3-(4-hydroxyphenyl)propanoic acid amide

-
C
-
83345-46-4
N-(tert-butyloxycarbonyl)-L-tyrosinol

| Conditions | Yield |
|---|---|
| With sodium; ammonium chloride In ammonia | A 8% B 12% C n/a |


| Conditions | Yield |
|---|---|
| With ammonia; water | |
| With diiron nonacarbonyl In benzene |
-
-
24608-52-4
tert-butyl chloroformate

-
-
4248-19-5
tert-butyl carbamate

| Conditions | Yield |
|---|---|
| With ammonia |

| Conditions | Yield |
|---|---|
| With water |
-
-
57022-34-1
tert-butyl cyanoformate

-
-
4248-19-5
tert-butyl carbamate

| Conditions | Yield |
|---|---|
| With ammonium hydroxide |
-
-
99768-94-2
3-Brom-propionylcarbamidsaeure-tert.-butylester

-
-
4248-19-5
tert-butyl carbamate

| Conditions | Yield |
|---|---|
| With sodium hydroxide |

| Conditions | Yield |
|---|---|
| In Petroleum ether |
-
-
507-40-4
tert-butylhypochlorite

-
-
4203-28-5
phenyl-diazenecarboxylic acid amide

-
-
4248-19-5
tert-butyl carbamate

| Conditions | Yield |
|---|---|
| In chloroform |

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In benzene |
-
-
88463-18-7
(S)-2-[(tert-butoxycarbonyl)amino]-3-phenylpropionamide

-
A
-
4248-19-5
tert-butyl carbamate

-
B
-
102-93-2
3-phenylpropionamide

-
C
-
66605-57-0
(S)-N-tert-butoxycarbonyl-2-amino-3-phenylpropanol

| Conditions | Yield |
|---|---|
| With sodium; ammonium chloride In ammonia |

-
-
78497-71-9
N-(1-aminoisobityl)carbamic acid tert-butyl ester hydrochloride

-
A
-
4248-19-5
tert-butyl carbamate

-
B
-
7664-41-7
ammonia

-
C
-
78-84-2
isobutyraldehyde

| Conditions | Yield |
|---|---|
| With phosphoric acid at 40 - 45℃; Product distribution; Rate constant; Mechanism; influence of pH; |

-
-
83351-75-1
tert-butyl (S)-(1-amino-3-(4-hydroxyphenyl)-1-oxopropan-2-yl)carbamate

-
A
-
4248-19-5
tert-butyl carbamate

-
B
-
102-93-2
3-phenylpropionamide

-
C
-
66605-57-0
(S)-N-tert-butoxycarbonyl-2-amino-3-phenylpropanol

| Conditions | Yield |
|---|---|
| With sodium; ammonium chloride In ammonia |

-
-
98115-12-9
Nα,Nca-di-tert-butyloxycarbonylasparagine

-
A
-
4248-19-5
tert-butyl carbamate

-
B
-
13726-67-5
Boc-Asp-OH

| Conditions | Yield |
|---|---|
| With sodium hydroxide |
-
-
98115-14-1
Nα,Nca-di-tert-butyloxycarbonylglutamine

-
A
-
4248-19-5
tert-butyl carbamate

-
B
-
2419-94-5
Boc-Glu

| Conditions | Yield |
|---|---|
| With sodium hydroxide |

| Conditions | Yield |
|---|---|
| With diethyl ether |

-
-
73017-98-8
N-<(tert-butoxycarbonyl)amino>methylamine hydrochloride

-
A
-
50-00-0
formaldehyd

-
B
-
4248-19-5
tert-butyl carbamate

| Conditions | Yield |
|---|---|
| With water-d2 Rate constant; |


| Conditions | Yield |
|---|---|
| With sodium hydroxide In tetrahydrofuran; methanol; water | 28.11 g (68%) |
-
-
50903-59-8
N-t-butyloxycarbonyl-Nε-benzyloxycarbonyl-lysine pentafluorophenyl ester

-
-
4248-19-5
tert-butyl carbamate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 90.5 percent / 25percent NH4OH / dioxane / 1 h 2: 94 percent / 1) 10percent Pd/C, H2 / methanol / Ambient temperature; 1) 1 h 3: 14.7 percent / Na, liq. NH3 View Scheme |
-
-
55592-81-9
Nε-(benzyloxycarbonyl)-Nα-(tert-butoxycarbonyl)-L-lysinamide

-
-
4248-19-5
tert-butyl carbamate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 94 percent / 1) 10percent Pd/C, H2 / methanol / Ambient temperature; 1) 1 h 2: 14.7 percent / Na, liq. NH3 View Scheme |
-
-
35454-04-7
oxalamic Acid Tert-butyl Ester

-
-
4248-19-5
tert-butyl carbamate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: (CF3CO)2O / pyridine 2: aq. NH3 View Scheme |
-
-
4248-19-5
tert-butyl carbamate

-
-
54957-94-7
tert-butyl N,N-dichlorocarbamate

| Conditions | Yield |
|---|---|
| With hydrogenchloride; calcium hypochlorite In dichloromethane; water at 0 - 1℃; for 0.916667h; | 100% |
| With sodium hypochlorite; acetic acid at 0 - 5℃; for 0.266667h; | 86% |
| With hydrogenchloride; calcium hypochlorite In dichloromethane; water at 0℃; for 1.75h; regiospecific reaction; | 84% |
| With hydrogenchloride; calcium hypochlorite In dichloromethane |
-
-
4248-19-5
tert-butyl carbamate

-
-
298-12-4
Glyoxilic acid

-
-
96625-24-0
Nα-(tert-butoxycarbonyl)-α-hydroxyglycine

| Conditions | Yield |
|---|---|
| In acetone for 4h; Heating; | 100% |
| In diethyl ether for 72h; Ambient temperature; | 2.5 g |
| In diethyl ether for 72h; Ambient temperature; | |
| In acetone for 5h; Heating; | |
| In acetone Heating; |
-
-
4248-19-5
tert-butyl carbamate

-
-
873-55-2
sodium benzenesulfonate

-
-
100-52-7
benzaldehyde

-
-
155396-71-7
tert-butyl N-(phenyl(phenylsulfonyl)methyl)carbamate

| Conditions | Yield |
|---|---|
| With formic acid In methanol; water at 20℃; for 72h; | 100% |
| In methanol; formic acid; water at 20℃; for 72h; | 99% |
| In methanol; formic acid; water at 20℃; for 72h; | 98% |

-
-
4248-19-5
tert-butyl carbamate

-
-
358365-86-3
tert-butyl N,N-dibromocarbamate

| Conditions | Yield |
|---|---|
| With bromine; sodium hydroxide In water at 20℃; for 2h; | 100% |
| With bromine; potassium carbonate In water at 20℃; for 2h; |
-
-
446026-44-4
4-benzyloxy-3-formyl-benzonitrile

-
-
4248-19-5
tert-butyl carbamate

-
-
758710-64-4
4-benzyloxy-3-tert-butoxycarbonylaminomethylbenzonitrile

| Conditions | Yield |
|---|---|
| With triethylsilane; trifluoroacetic acid In acetonitrile at 20℃; for 2h; | 100% |

| Conditions | Yield |
|---|---|
| With caesium carbonate; 4,5-bis-(di-tert-butyl-phosphanyl)-9,9-dimethyl-9H-xanthene; palladium diacetate In 1,4-dioxane at 110℃; for 16h; | 100% |

-
-
4248-19-5
tert-butyl carbamate

-
-
891772-49-9
7-oxo-3a,7,8,8a-tetrahydro-1H,3H-2,7a-diaza-cyclopenta[a]indene-2-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With hydrogen; palladium(II) hydroxide/carbon In methanol at 50℃; under 2327.23 Torr; for 3h; | 100% |
-
-
4248-19-5
tert-butyl carbamate

-
-
1146732-04-8
3-[6'-chloro-4'-(3,4-dimethyl-2-oxo-2,3-dihydro-benzoxazole-6-carbonyl)-3,4,5,6-tetrahydro-2H-[1,2']bipyridinyl-4-yl]-7-methoxy-1,3,4,5-tetrahydro-benzo[d][1,3]diazepin-2-one

-
-
1146733-74-5
tert-butyl [4'-(3,4-dimethyl-2-oxo-2,3-dihydro-benzoxazole-6-carbonyl)-4-(7-methoxy-2-oxo-1,2,4,5-tetrahydro-benzo[d][1,3]diazepin-3-yl)-3,4,5,6-tetrahydro-2H-[1,2']bipyridinyl-6'-yl]-carbamate

| Conditions | Yield |
|---|---|
| With caesium carbonate; 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene; tris-(dibenzylideneacetone)dipalladium(0) In 1,4-dioxane for 15h; Reflux; | 100% |
-
-
4248-19-5
tert-butyl carbamate

-
-
1365992-18-2
4-(4-bromothiazolo[5,4-c]pyridin-2-yl)-3-chloro-5-fluorobenzonitrile

-
-
1365992-31-9
[2-(2-chloro-4-cyano-6-fluorophenyl)thiazolo[5,4-c]pyridin-4-yl]-carbamic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With potassium phosphate; tris-(dibenzylideneacetone)dipalladium(0); 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene In water; toluene at 60℃; for 4h; Inert atmosphere; | 100% |
-
-
4248-19-5
tert-butyl carbamate

-
-
1380331-16-7
4-(7-bromo-[1,2,4]triazolo[1,5-a]pyridine-2-yl)morpholine

-
-
1380331-17-8
tert-butyl 2-morpholino-[1,2,4]triazolo[1,5-a]pyridin-7-ylcarbamate

| Conditions | Yield |
|---|---|
| With caesium carbonate; tris-(dibenzylideneacetone)dipalladium(0); 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene In 1,4-dioxane at 110℃; for 20h; Inert atmosphere; Sealed tube; | 100% |
| With caesium carbonate; tris-(dibenzylideneacetone)dipalladium(0); 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene In 1,4-dioxane at 110℃; for 20h; Inert atmosphere; |
-
-
4248-19-5
tert-butyl carbamate

-
-
1279461-93-6
5-((5-bromopyridin-3-yl)oxy)pyrimidine

-
-
1279461-97-0
tert-butyl (5-(pyrimidin-5-yloxy)pyridin-3-yl)carbamate

| Conditions | Yield |
|---|---|
| With sodium t-butanolate; tert-butyl XPhos; tris(dibenzylideneacetone)dipalladium(0) chloroform complex In toluene at 20℃; | 100% |
-
-
940289-71-4
2,2,5-trimethyl-4-oxo-4H-1,3-benzodioxin-7-yl trifluoromethanesulfonate

-
-
4248-19-5
tert-butyl carbamate

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); caesium carbonate; 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene In 1,4-dioxane at 80℃; for 5h; Inert atmosphere; | 100% |
-
-
4248-19-5
tert-butyl carbamate

| Conditions | Yield |
|---|---|
| With lithium tert-butoxide In tetrahydrofuran at -23 - 20℃; for 48h; Inert atmosphere; | 100% |
-
-
4248-19-5
tert-butyl carbamate

| Conditions | Yield |
|---|---|
| With lithium tert-butoxide In tetrahydrofuran at -23 - 20℃; for 48h; Inert atmosphere; | 100% |
-
-
4248-19-5
tert-butyl carbamate

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); caesium carbonate; XPhos In 1,4-dioxane at 80℃; for 2h; Inert atmosphere; | 100% |
-
-
4248-19-5
tert-butyl carbamate

| Conditions | Yield |
|---|---|
| With palladium diacetate; caesium carbonate; XPhos In 1,4-dioxane at 95℃; for 18h; | 100% |

-
-
4248-19-5
tert-butyl carbamate

-
-
301666-75-1
ethyl 5-((tert-butoxycarbonyl)amino)-2-methylnicotinate

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); caesium carbonate; 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene In 1,4-dioxane at 85℃; for 18h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With triethylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In dichloromethane at 20℃; for 3h; | 100% |
-
-
4248-19-5
tert-butyl carbamate

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); sodium t-butanolate; tert-butyl XPhos In toluene for 16h; Sealed tube; | 100% |

-
-
4248-19-5
tert-butyl carbamate

| Conditions | Yield |
|---|---|
| Stage #1: tert-butyl carbazate With potassium hexamethylsilazane In tetrahydrofuran at -78℃; for 0.5h; Inert atmosphere; Stage #2: (S)-2-methyl-1-[(2-nitrophenyl)sulfonyl]aziridine In tetrahydrofuran; dichloromethane at 20℃; for 18h; Inert atmosphere; | 100% |
-
-
4248-19-5
tert-butyl carbamate


| Conditions | Yield |
|---|---|
| With triethylsilane; trifluoroacetic acid In dichloromethane at 0 - 20℃; Inert atmosphere; | 100% |
-
-
4248-19-5
tert-butyl carbamate

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); 1,1'-bis(di-tertbutylphosphino)ferrocene; potassium carbonate In toluene at 75℃; for 6h; Inert atmosphere; | 100% |
-
-
18292-38-1
2-methallyltrimethylsilane

-
-
4248-19-5
tert-butyl carbamate

-
-
913388-24-6
tert-butyl 5-(dimethoxymethyl)-1H-indole-1-carboxylate

| Conditions | Yield |
|---|---|
| With triethylsilyl trifluoromethyl sulfonate; C35H31F6N3OS In diethyl ether at -78 - -50℃; for 6h; Catalytic behavior; Reagent/catalyst; Inert atmosphere; Sealed tube; enantioselective reaction; | 100% |

-
-
1745-46-6
6,11-dihydrodibenzo[b,e]thiepin-11-ol

-
-
4248-19-5
tert-butyl carbamate

-
-
51065-32-8
(5,11-Dihydro-10-thia-dibenzo[a,d]cyclohepten-5-yl)-carbamic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In acetic acid 1) 37 deg C, 6h; 2) room temp., overnight; | 99% |
-
-
4248-19-5
tert-butyl carbamate

| Conditions | Yield |
|---|---|
| With N-chloro-succinimide; isopropylamine In methanol for 2h; Ambient temperature; | 99% |
-
-
104-92-7
1-bromo-4-methoxy-benzene

-
-
4248-19-5
tert-butyl carbamate

-
-
18437-68-8
tert-butyl N-(4-methoxyphenyl)carbamate

| Conditions | Yield |
|---|---|
| With di-tert-butyl(2,2-diphenyl-1-methyl-1-cyclopropyl)phosphine; bis(η3-allyl-μ-chloropalladium(II)); sodium t-butanolate In water at 50℃; for 24h; Inert atmosphere; Green chemistry; | 99% |
| With di-tert-butyl{2′-isopropoxy-[1,1′-binaphthalen]-2-yl}phosphane; caesium carbonate; bis(dibenzylideneacetone)-palladium(0) In tert-butyl alcohol at 100℃; for 20h; Inert atmosphere; | 98% |
| With tris(dibenzylideneacetone)dipalladium(0) chloroform complex; sodium t-butanolate; tert-butyl XPhos In toluene at 17 - 22℃; for 30h; Inert atmosphere; | 76% |
-
-
1436-34-6
1,2-Epoxyhexane

-
-
4248-19-5
tert-butyl carbamate

-
A
-
1436-34-6, 122922-40-1, 130404-08-9, 104898-06-8
(R)-1,2-epoxyhexane

| Conditions | Yield |
|---|---|
| With air; 4-nitro-benzoic acid; (R,R)-((t-Bu)4-salen)Co(II) In various solvent(s) at 20℃; for 24h; | A n/a B 99% |
| With (1R,2R)-(-)-N,N'-bis(3,5-di-tert-butylsalicydene)-1,2-cyclohexanediaminocobalt(II); 3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate; 4-nitro-benzoic acid at 20℃; for 10h; optical yield given as %ee; enantioselective reaction; |

-
-
52485-73-1, 105205-70-7, 126872-17-1, 19600-63-6
1,2-epoxy-7-octene

-
-
4248-19-5
tert-butyl carbamate

-
A
-
105205-70-7
(R)-1,2-epoxy-7-octene

| Conditions | Yield |
|---|---|
| With air; 4-nitro-benzoic acid; (R,R)-((t-Bu)4-salen)Co(II) In various solvent(s) at 20℃; for 24h; | A n/a B 99% |

-
-
4248-19-5
tert-butyl carbamate

-
-
122-60-1
Phenyl glycidyl ether

-
A
-
71031-03-3
(2R)-1,2-epoxy-3-phenoxypropane

| Conditions | Yield |
|---|---|
| With air; 4-nitro-benzoic acid; [(S,S)-N,N’-bis(3,5-di-tertbutylsalicylidene)-1,2-cyclohexanediaminato(2-)]cobalt(II) In various solvent(s) at 20℃; for 24h; | A n/a B 99% |

-
-
4248-19-5
tert-butyl carbamate

-
-
1196-90-3
4-bromo-1-methyl-1H-pyrrole-2-carboxylic acid methyl ester

-
-
126092-96-4
methyl 4-<<(tert-butyloxy)carbonyl>amino>-1-methyl-pyrrole-2-carboxylate

| Conditions | Yield |
|---|---|
| With potassium phosphate; N,N`-dimethylethylenediamine; copper(l) iodide In 1,4-dioxane at 110℃; for 48h; | 99% |
Tert-butyl carbamate Specification
The tert-Butyl carbamate, with the CAS registry number 4248-19-5, is also known as Carbamic acid, 1,1-dimethylethyl ester. It belongs to the product categories of Protected Amino Acids; Pharmacetical. Its EINECS registry number is 224-209-3. This chemical's molecular formula is C5H11NO2 and molecular weight is 117.15. What's more, its systematic name is called 2-Methyl-2-propanyl carbamate. It should be stored in a cool, dry and well-ventilated place. This chemical can be prepared by tertiary butanol with sodium cyanate. This reaction needs reagent Trifluoroacetic acid and solvent benzene.
Physical properties about tert-Butyl carbamate are: (1)ACD/LogP: 0.57; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0.57; (4)ACD/LogD (pH 7.4): 0.57; (5)ACD/BCF (pH 5.5): 1.60; (6)ACD/BCF (pH 7.4): 1.60; (7)ACD/KOC (pH 5.5): 48.66; (8)ACD/KOC (pH 7.4): 48.66; (9)#H bond acceptors: 3; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 2; (12) Polar Surface Area: 52.32 Å2; (13)Index of Refraction: 1.429; (14)Molar Refractivity: 30.489 cm3; (15)Molar Volume: 118.277 cm3; (16)Polarizability: 12.087×10-24 cm3; (17)Surface Tension: 31.38 dyne/cm; (18)Density: 0.99 g/cm3; (19)Flash Point: 82.407 °C; (20)Enthalpy of Vaporization: 43.218 kJ/mol; (21)Boiling Point: 195.979 °C at 760 mmHg; (22)Vapour Pressure: 0.408 mmHg at 25 °C.
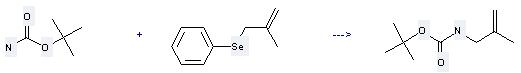
Uses of tert-Butyl carbamate: (1) it is used as organic synthetic reagent and pharmaceutical intermediates; (2) it is used to produce other chemicals. For example, it can react with Methallyl(phenyl)selenid to get N-(tert-butoxycarbonyl)-2-methyl-2-propenylamine. This reaction needs reagent diisopropylethylamine / N-chlorosuccinimide and solvent methanol at temperature of 0 °C. The yield is 92 %.
When you are dealing with this chemical, you should be very careful. This chemical is irritating to eyes and may cause inflammation to the skin or other mucous membranes. Therefore, you should wear suitable eye/face protection. In case of contacting with eyes, you should rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1) SMILES: O=C(OC(C)(C)C)N
(2) InChI: InChI=1S/C5H11NO2/c1-5(2,3)8-4(6)7/h1-3H3,(H2,6,7)
(3) InChIKey: LFKDJXLFVYVEFG-UHFFFAOYSA-N
Related Products
- tert-Butyl (1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)acetate
- tert-Butyl (1-formylcyclopropyl)carbamate
- tert-Butyl (1-hydroxy-3-phenylpropan-2-yl)carbamate
- tert-Butyl (1S,4S)-2,5-diazabicyclo[2.2.1]heptan-2-carboxylate
- tert-Butyl (2-aminophenyl)carbamate
- tert-Butyl (2-chloro-3-formylpyridin-4-yl)carbamate
- tert-Butyl (2R)-2-(hydroxymethyl)-5-oxopyrrolidine-1-carboxylate
- tert-Butyl (2R,3S)-(-)-6-oxo-2,3-diphenyl-4-morpholinecarboxylate
- tert-Butyl (2S)-2-(hydroxymethyl)-5-oxopyrrolidine-1-carboxylate
- tert-Butyl (2S)-2-carbamoyl-2,3-dihydropyrrole-1-carboxylate
- 42482-06-4
- 424823-02-9
- 42484-95-7
- 42486-84-0
- 42487-07-0
- 42487-09-2
- 42487-22-9
- 4248-74-2
- 42489-42-9
- 4249-10-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View