-
Name
Tosyl chloride
- EINECS 202-684-8
- CAS No. 98-59-9
- Article Data183
- CAS DataBase
- Density 1.339 g/cm3
- Solubility hydrolyses in water
- Melting Point 65-69 °C(lit.)
- Formula C7H7ClO2S
- Boiling Point 265.3 °C at 760 mmHg
- Molecular Weight 190.65
- Flash Point 114.3 °C
- Transport Information UN 3261 8/PG 2
- Appearance White to yellow solid
- Safety 26-36/37/39-45-27
- Risk Codes 34-29
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms 4-Methylbenzene-1-sulfonyl chloride;4-Methylbenzenesulfonylchloride;4-Methylphenylsulfonyl chloride;4-Toluenesulfonyl chloride;4-Toluensulfonyl chloride;4-Toluolsulfonyl chloride;4-Tosyl chloride;NSC175822;Toluenesulfonyl chloride;p-Methylbenzenesulfonylchloride;p-Methylphenylsulfonyl chloride;p-Toluenesulfochloride;p-Toluenesulfonic acid chloride;p-Toluenesulfonic chloride;p-Toluenesulphonylchloride;p-Tolylsulfonyl chloride;p-Tosyl chloride;p-Toluenesulfonylchloride (8CI);
- PSA 42.52000
- LogP 3.00330
Synthetic route

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; zirconium(IV) chloride In water; acetonitrile at 25℃; for 0.0166667h; | 99% |
| With N,N,N',N'-tetrachlorobenzene-1,3-disulphonamide; tetrabutyl-ammonium chloride; water In acetonitrile at 0 - 20℃; for 0.333333h; | 98% |
| With trichloroisocyanuric acid; water In acetonitrile at 0 - 20℃; for 0.333333h; | 98% |
-
-
15404-00-9
triethylammonium toluene-p-sulfonate

-
-
98-59-9
p-toluenesulfonyl chloride

| Conditions | Yield |
|---|---|
| With sulfuryl dichloride; triphenylphosphine In dichloromethane for 1h; Ambient temperature; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: p-toluidine With hydrogenchloride; acetic acid; sodium nitrite In water; acetonitrile at 0 - 5℃; Stage #2: With sulfur dioxide; copper dichloride In water; acetonitrile at 20℃; for 16h; | 99% |
| Stage #1: p-toluidine With hydrogenchloride; sodium nitrite Stage #2: With sulfur dioxide; copper(l) chloride In acetone |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; zirconium(IV) chloride In water; acetonitrile at 25℃; for 0.0166667h; | 98% |
| With Oxone; potassium chloride In water at 20℃; for 0.166667h; | 96% |
| With N,N,N',N'-tetrachlorobenzene-1,3-disulphonamide; tetrabutyl-ammonium chloride; water In acetonitrile at 0 - 20℃; for 0.333333h; | 95% |

| Conditions | Yield |
|---|---|
| With 1,3,5-trichloro-2,4,6-triazine; triethylamine In acetone for 20h; Heating; | 96% |
| Stage #1: toluene-4-sulfonic acid With potassium phosphate; bis(trichloromethyl) carbonate In dichloromethane at 0 - 5℃; for 0.0833333h; Stage #2: With triethylamine In dichloromethane at 20℃; for 0.5h; | 96% |
| With 2,2,4,4,6,6-hexachloro-1,3,5-triaza-2,4,6-triphosphorine; potassium chloride at 25℃; for 0.0333333h; Neat (no solvent); | 94% |
-
-
146404-41-3
C15H17ClN2O2S

-
A
-
32327-70-1
1-chloro-4-(2-chloroethyl)benzene

-
B
-
98-59-9
p-toluenesulfonyl chloride

| Conditions | Yield |
|---|---|
| With N-chloro-succinimide In tetrahydrofuran for 16h; Ambient temperature; Irradiation; | A 95% B n/a |

| Conditions | Yield |
|---|---|
| With 1,3,5-trichloro-2,4,6-triazine; 18-crown-6 ether In acetone for 20h; Heating; | 94% |
| Stage #1: sodium tosylate With potassium phosphate; bis(trichloromethyl) carbonate In dichloromethane at 0 - 5℃; for 0.0833333h; Stage #2: With triethylamine In dichloromethane at 20℃; for 0.5h; | 93% |
| With trichlorophosphate In sulfolane; acetonitrile at 68 - 72℃; for 0.666667h; | 87% |

| Conditions | Yield |
|---|---|
| With chlorine In water; toluene Product distribution / selectivity; | 93.9% |
-
-
37091-73-9
2-chloro-1,3-dimethylimidazolinium chloride

-
-
98-59-9
p-toluenesulfonyl chloride

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene | 93.2% |
-
-
4358-63-8
phenyl benzenesulfonate

-
-
5720-05-8
4-methylphenylboronic acid

-
-
98-59-9
p-toluenesulfonyl chloride

| Conditions | Yield |
|---|---|
| With phenylchlorosulfate; C43H43NO3PPdS; sodium carbonate In acetone at 20 - 50℃; Inert atmosphere; Sealed tube; | 93% |

| Conditions | Yield |
|---|---|
| With chlorosulfonic acid at 0℃; for 4h; | 92% |
| With chlorosulfonic acid at 0 - 20℃; | 84.21% |
| With chlorosulfonic acid |
-
-
61124-61-6
3-phenylpropanal p-tosylhydrazone

-
B
-
80491-28-7
5-Benzyl-1,2,3-thiadiazole

-
C
-
98-59-9
p-toluenesulfonyl chloride

| Conditions | Yield |
|---|---|
| With disulfur dichloride In dichloromethane at 20℃; for 0.5h; Product distribution; other reagents (SOCl2, SCl2) and time; | A n/a B 89% C n/a |

-
-
146404-40-2
1-tosyl-1-hexadecylhydrazine

-
A
-
4860-03-1
1-Chlorohexadecan

-
B
-
98-59-9
p-toluenesulfonyl chloride

| Conditions | Yield |
|---|---|
| With N-chloro-succinimide In tetrahydrofuran for 16h; Ambient temperature; Irradiation; | A 88% B n/a |
| With N-chloro-succinimide In tetrahydrofuran for 16h; Product distribution; Mechanism; Ambient temperature; Irradiation; reactions of N-substituted-N-tosylhydrazines with NCS and NBS; | A 88% B n/a |
-
-
5362-76-5
mesityl oxide p-tosylhydrazone

-
A
-
70-55-3
toluene-4-sulfonamide

-
B
-
98-59-9
p-toluenesulfonyl chloride

| Conditions | Yield |
|---|---|
| With sulfur dichloride In dichloromethane at 20℃; for 0.5h; Product distribution; other reagents (SOCl2, S2Cl2), products and time; | A n/a B n/a C 9% D 85% |

-
-
1666-13-3
diphenyl diselenide

-
-
1576-35-8
toluene-4-sulfonic acid hydrazide

-
A
-
68819-94-3
p-tolyl benzeneselenosulfonate

-
B
-
98-59-9
p-toluenesulfonyl chloride

| Conditions | Yield |
|---|---|
| With sulfuryl dichloride; triethylamine In pyridine; dichloromethane at 0℃; Product distribution; various reagents for preparation of diverse selenosulfonates; mechanism is proposed; | A 83% B 11% |

-
-
1950-78-3
p-toluenesulfonyl iodide

-
-
981-18-0
triphenyl(phenylazo)methane

-
A
-
591-50-4
iodobenzene

-
B
-
2943-42-2
di(4-methyl)phenylthiosulfonate

-
C
-
4124-41-8
p-toluenesulfonylanhydride

-
D
-
42756-18-3
p-tolyl triphenylmethyl sulphone

-
E
-
108-90-7
chlorobenzene

-
F
-
98-59-9
p-toluenesulfonyl chloride

| Conditions | Yield |
|---|---|
| In tetrachloromethane at 0℃; for 2h; Product distribution; | A 82% B n/a C n/a D 78% E 1% F n/a |

-
-
141666-87-7
1-Tosyl-1-<2-(4-methoxyphenyl)ethyl>hydrazine

-
A
-
98-59-9
p-toluenesulfonyl chloride

-
B
-
18217-00-0
1-(2-Chloroethyl)-4-methoxybenzene

| Conditions | Yield |
|---|---|
| With N-chloro-succinimide In tetrahydrofuran for 16h; Ambient temperature; Irradiation; | A n/a B 82% |

| Conditions | Yield |
|---|---|
| With tert-butylhypochlorite In dichloromethane for 2h; Ambient temperature; chloramine-T, CH2Cl2, RT; | 81% |
| With water; chlorine | |
| With N-chloro-succinimide In tetrahydrofuran Ambient temperature; Irradiation; Yield given; | |
| Multi-step reaction with 2 steps 1: 81 percent / CH2Cl2 / 2 h / Ambient temperature 2: 1.) n-butyllithium, 2.) SO2Cl2 / 1.) THF, hexane, -78 deg C, 2.) -78 deg C, 20 min View Scheme |

| Conditions | Yield |
|---|---|
| In pyridine 1)50 deg C, 2h 2)room temp., 20h; | A 13% B 81% |

| Conditions | Yield |
|---|---|
| With chlorosulfonic acid; sodium chloride at -0.16℃; | A 80% B 20% |
| With chlorosulfonic acid for 3h; Cooling with ice; | A n/a B 72.04% |
| With sulfuric acid; sodium chloride Edukt 4: SO3; |
-
-
41780-85-2
1-acetylcyclohexene p-tosylhydrazone

-
A
-
70-55-3
toluene-4-sulfonamide

-
B
-
98-59-9
p-toluenesulfonyl chloride

| Conditions | Yield |
|---|---|
| With sulfur dichloride In dichloromethane at 20℃; for 0.5h; Product distribution; other reagents (SOCl2, S2Cl2) and time; | A n/a B n/a C 80% |


| Conditions | Yield |
|---|---|
| With potassium carbonate | 77% |
-
-
23328-69-0
4-chloromorpholine

-
-
536-57-2
p-toluene sulfinic acid

-
A
-
6339-26-0
4-tosylmorpholine

-
B
-
98-59-9
p-toluenesulfonyl chloride

| Conditions | Yield |
|---|---|
| In dichloromethane for 0.5h; Ambient temperature; | A 76% B 10% |


| Conditions | Yield |
|---|---|
| With pyridine; (Dichloroiodo)benzene In 1,2-dichloro-ethane at 20 - 88℃; for 0.0166667h; Inert atmosphere; Reflux; | 74% |

-
B
-
98-59-9
p-toluenesulfonyl chloride

| Conditions | Yield |
|---|---|
| A 73% B n/a |
-
-
56410-24-3
N,N'-bis(p-toluenesulfonyl)hydroxylamine

-
A
-
62419-04-9
bis(4-methylphenylsulfonyl)amino 4-methylbenzenesulfonate

-
B
-
104-15-4
toluene-4-sulfonic acid

-
C
-
98-59-9
p-toluenesulfonyl chloride

| Conditions | Yield |
|---|---|
| With air In chloroform for 12h; Product distribution; Mechanism; Ambient temperature; various reaction conditions; | A 20% B 70% C 5% |


| Conditions | Yield |
|---|---|
| With N-chloro-succinimide In tetrahydrofuran for 16h; Ambient temperature; Irradiation; | A n/a B 68% |
-
-
1950-69-2
toluene-p-sulfonyl bromide

-
-
981-18-0
triphenyl(phenylazo)methane

-
A
-
108-86-1
bromobenzene

-
B
-
2943-42-2
di(4-methyl)phenylthiosulfonate

-
C
-
42756-18-3
p-tolyl triphenylmethyl sulphone

-
D
-
108-90-7
chlorobenzene

-
E
-
98-59-9
p-toluenesulfonyl chloride

| Conditions | Yield |
|---|---|
| In tetrachloromethane for 30h; Product distribution; Heating; | A 67% B n/a C 56% D 6% E n/a |

-
-
10439-23-3
4-methylbenzenesulfinyl chloride

-
-
2696-32-4
but-3-en-1-ynyl-trimethyl-silane

-
A
-
98-59-9
p-toluenesulfonyl chloride

| Conditions | Yield |
|---|---|
| aluminium trichloride In diethyl ether Ambient temperature; | A n/a B 66% C n/a |

-
-
97-99-4
Tetrahydrofurfuryl alcohol

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
34583-63-6
rac-(tetrahydrofuran-2-yl)methyl 4-methylbenzenesulfonate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0℃; for 1h; | 100% |
| With potassium hydroxide | 93% |
| With potassium iodide; silver(l) oxide In dichloromethane at 40℃; | 91% |
-
-
504-29-0
2-aminopyridine

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
52776-76-8
N-(2-pyridyl)-p-toluenesulfonamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 4℃; | 100% |
| at 20℃; for 0.1h; | 88% |
| With sodium carbonate In water | 82.1% |
-
-
578-66-5
8-amino quinoline

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
10304-39-9
4-methyl-N-quinolin-8-yl-benzenesulfonamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 4℃; | 100% |
| With pyridine at 130℃; for 0.05h; Microwave irradiation; Inert atmosphere; | 97% |
| With pyridine at 130℃; for 0.166667h; | 90% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 2h; | 100% |
| With triethylamine In dichloromethane at 0 - 20℃; | 99% |
| With triethylamine In dichloromethane at 0 - 20℃; for 2h; | 99% |

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 20℃; for 28h; | 100% |
| With triethylamine In dichloromethane at 4℃; | 100% |
| With triethylamine In tetrahydrofuran at 20℃; for 15h; | 95% |
-
-
551-93-9
2-aminoacetophenone

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
1859-70-7
N-(2-acetylphenyl)-4-methylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 0 - 20℃; for 10.5h; | 100% |
| With triethylamine In dichloromethane at 4℃; | 100% |
| With pyridine In dichloromethane at 20℃; for 1h; | 97% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 4℃; | 100% |
| With pyridine | 98% |
| at 20℃; for 0.166667h; | 94% |
-
-
92-67-1
4-Aminobiphenyl

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
65690-69-9
N-([1,1'-biphenyl]-4-yl)-4-methylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 4℃; | 100% |
| With pyridine at 0 - 20℃; for 15h; Inert atmosphere; | 75% |
| With pyridine |
-
-
108-91-8
cyclohexylamine

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
80-30-8
N-cyclohexyl-p-toluenesulfonamide

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 0.5h; Inert atmosphere; | 100% |
| With pyridine at 0 - 25℃; Inert atmosphere; Schlenk technique; | 99% |
| With triethylamine In tetrahydrofuran at 0 - 20℃; | 95% |
-
-
106-49-0
p-toluidine

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
599-86-0
4-methyl-N-(4-methylphenyl)benzenesulfonamide

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 20℃; for 16h; | 100% |
| With pyridine at 0 - 25℃; | 100% |
| In water at 110℃; for 0.0833333h; Microwave irradiation; Green chemistry; chemoselective reaction; | 98% |
-
-
106-47-8
4-chloro-aniline

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
2903-34-6
4-methyl-N-(4-chlorophenyl)benzenesulfonamide

| Conditions | Yield |
|---|---|
| In pyridine; acetonitrile at 20℃; for 16h; | 100% |
| In ethanol; water at 25℃; for 0.333333h; | 96% |
| With (Na1752K0.144Ca0365Mg0.065)(Al2044Si2774O96)*19.16H2O In ethanol at 25 - 30℃; for 0.25h; Sonication; Green chemistry; | 95% |
-
-
104-94-9
4-methoxy-aniline

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
1150-26-1
N-(4-Methoxy-phenyl)-4-methyl-benzenesulfonamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 4℃; | 100% |
| With pyridine for 16h; Reflux; | 97% |
| With dendritic fibrous nanosilica KCC-1 3-aminopropyl-functionalized supported on Fe3O4 magnetic nanocatalyst In water at 20℃; for 0.5h; Solvent; Temperature; Reagent/catalyst; Time; Green chemistry; | 97% |
-
-
90-41-5
2-phenylaniline

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
24310-30-3
N-biphenyl-2-yl-4-methylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 4℃; | 100% |
| With sodium hydrogencarbonate In water; acetone at 25℃; for 0.25h; | 97% |
| With pyridine at 0 - 25℃; for 1h; | 92% |
-
-
141-43-5
ethanolamine

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
14316-14-4
N-(2-hydroxy-ethyl)-4-methyl-benzenesulfonamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 18h; Inert atmosphere; | 100% |
| With triethylamine In dichloromethane at 0 - 20℃; for 24h; | 99% |
| With pyridine at 5 - 20℃; | 96% |
-
-
110-58-7
1-pentanamine

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
106011-68-1
4-methyl-N-pentylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane at 0 - 20℃; for 12h; | 100% |
| With dmap; triethylamine In dichloromethane at 0 - 20℃; for 2h; Inert atmosphere; | 89% |
| With triethylamine In dichloromethane at 0℃; for 0.0833333h; | 85% |
-
-
107-19-7
propargyl alcohol

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
6165-76-0
propargyl p-toluenesulfonate

| Conditions | Yield |
|---|---|
| With sodium hydroxide In diethyl ether; water at 0 - 20℃; for 17h; | 100% |
| With potassium hydroxide In diethyl ether at -5 - 20℃; | 96% |
| With potassium hydroxide In diethyl ether at -5 - 0℃; for 0.5h; | 95% |
-
-
108-69-0
3,5-dimethylaminoaniline

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
114097-28-8
N-(3,5-dimethylphenyl)-4-methyl-benzenesulfonamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 4℃; | 100% |
| With silica gel at 20℃; | 95% |
| With triethylamine In dichloromethane at 0 - 20℃; | 82% |
-
-
99-98-9
4-amino-N,N-dimethylaniline

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
19766-55-3
N-(4-(dimethylamino)phenyl)-4-methylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With pyridine for 1h; Reflux; | 100% |
| With caesium carbonate In acetonitrile at 25℃; for 0.333333h; | 93% |
| indium In acetonitrile at 20℃; for 6h; | 90% |
-
-
100-01-6
4-nitro-aniline

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
734-25-8
N-(4-nitrophenyl)-4-methylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 4℃; | 100% |
| With (Na1752K0.144Ca0365Mg0.065)(Al2044Si2774O96)*19.16H2O In ethanol at 25 - 30℃; for 2h; Sonication; Green chemistry; | 96% |
| With pyridine In dichloromethane at 20℃; for 12h; | 94% |
-
-
88-74-4
2-nitro-aniline

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
6380-13-8
4-methyl-N-(2-nitrophenyl)benzene sulfonamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 4℃; | 100% |
| With pyridine In dichloromethane at 20℃; for 48h; | 90% |
| With pyridine In dichloromethane at 20℃; for 16h; | 75% |
-
-
124-40-3
dimethyl amine

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
599-69-9
N,N,4-trimethylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 25℃; for 1h; | 100% |
| 99% | |
| With cesium fluoride supported on Celite at 50℃; for 0.75h; chemoselective reaction; | 91% |
-
-
75-31-0
isopropylamine

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
21230-07-9
N-isopropyl-p-toluenesulfonamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; | 100% |
| With pyridine; dmap In dichloromethane at 0 - 20℃; for 16h; | 99% |
| With pyridine at 0 - 25℃; Inert atmosphere; Schlenk technique; | 99% |

| Conditions | Yield |
|---|---|
| In pyridine at 20℃; | 100% |
| With pyridine at 0 - 20℃; | 95% |
| With sodium hydroxide In diethyl ether at 20℃; | 94% |
-
-
109-76-2
Trimethylenediamine

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
53364-99-1
N,N'-ditoluenesulfonyl-1,3-diaminopropane

| Conditions | Yield |
|---|---|
| With potassium carbonate In tetrahydrofuran; water at 0.2℃; for 3h; Inert atmosphere; | 100% |
| at 80℃; for 0.5h; | 95% |
| With potassium carbonate In tetrahydrofuran; water at 23℃; for 10h; | 95% |

| Conditions | Yield |
|---|---|
| With pyridine for 16h; Reflux; | 100% |
| With pyridine In dichloromethane at 20℃; for 16h; | 100% |
| With triethylamine In dichloromethane at 0 - 20℃; for 1h; Inert atmosphere; | 100% |
-
-
134-20-3
2-carbomethoxyaniline

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
50998-74-8
methyl N-tosylanthranilate

| Conditions | Yield |
|---|---|
| In pyridine at 25℃; | 100% |
| Stage #1: 2-carbomethoxyaniline With pyridine In dichloromethane at 25℃; for 1h; Inert atmosphere; Stage #2: p-toluenesulfonyl chloride In dichloromethane at 25℃; for 24h; Inert atmosphere; | 99% |
| In pyridine at 0℃; for 1h; | 98% |
-
-
110-80-5
2-ethoxy-ethanol

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
17178-11-9
2-ethoxyethyl p-toluenesulfonate

| Conditions | Yield |
|---|---|
| With pyridine at 0℃; | 100% |
| With sodium hydroxide In tetrahydrofuran; water | 85% |
| With triethylamine In dichloromethane at 20℃; for 4h; | 81.1% |
-
-
100-02-7
4-nitro-phenol

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
1153-45-3
4-nitrophenyl 4-methylbenzenesulfonate

| Conditions | Yield |
|---|---|
| Stage #1: 4-nitro-phenol; p-toluenesulfonyl chloride With potassium carbonate In acetone at 20 - 25℃; for 2.5h; Stage #2: With hydrogenchloride In water; acetone | 100% |
| With triethylamine In dichloromethane at 20℃; for 24h; Inert atmosphere; | 99% |
| With potassium carbonate for 0.0833333h; microwave irradiation; | 98% |
-
-
77-85-0
2-(hydroxymethyl)-2-methylpropane-1,3-diol

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
2387-43-1
2-methyl-2-((tosyloxy)methyl)propane-1,3-diyl bis(4-methylbenzenesulfonate)

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; for 16h; Inert atmosphere; | 100% |
| With pyridine at 0 - 20℃; | 90% |
| With pyridine In chloroform | 81% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-Phenoxyethanol; p-toluenesulfonyl chloride With dmap In dichloromethane at 20℃; Stage #2: With triethylamine In dichloromethane at 20℃; | 100% |
| With pyridine | 86% |
| With pyridine at 20℃; for 4h; | 24% |
Tosyl chloride Chemical Properties
IUPAC Name: 4-Methylbenzenesulfonyl chloride
Following is the structure of Tosyl chloride (CAS NO.98-59-9):
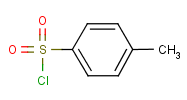
Empirical Formula: C7H7ClO2S
Molecular Weight: 190.6473
EINECS: 202-684-8
Index of Refraction: 1.545
Molar Refractivity: 45.02 cm3
Molar Volume: 142.3 cm3
Storage temp.: Store at RT.
Water Solubility: hydrolyses
Density: 1.339 g/cm3
Flash Point: 114.3 °C
Melting point: 65-69 °C(lit.)
Surface Tension: 41 dyne/cm
Enthalpy of Vaporization: 48.3 kJ/mol
Boiling Point: 265.3 °C at 760 mmHg
Vapour Pressure: 0.0151 mmHg at 25 °C
Appearance: White to yellow solid
Solubility of Tosyl chloride (CAS NO.98-59-9): Methylene chloride: 0.2 g/mL, clear
Product Categories of Tosyl chloride (CAS NO.98-59-9): Benzene derivatives; FINE Chemical & INTERMEDIATES; Protection & Derivatization Reagents (for Synthesis); Sulfur Compounds (for Synthesis); Synthetic Organic Chemistry
Canonical SMILES: CC1=CC=C(C=C1)S(=O)(=O)Cl
InChI: InChI=1S/C7H7ClO2S/c1-6-2-4-7(5-3-6)11(8,9)10/h2-5H,1H3
InChIKey: YYROPELSRYBVMQ-UHFFFAOYSA-N
Tosyl chloride Uses
Tosyl chloride (CAS NO.98-59-9) can be used as analytics reagents,and organic synthesis etc.
Tosyl chloride Safety Profile
Hazard Codes:  C
C
Risk Statements 34-29
R34:Causes burns.
R29:Contact with water liberates toxic gas.
Safety Statements 26-36/37/39-45-27
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S27:Take off immediately all contaminated clothing.
RIDADR: UN 3261 8/PG 2
WGK Germany: 1
F: 9-21
Hazard Note: Corrosive
HazardClass: 8
PackingGroup: II
HS Code: 29049020
Hazardous Substances Data: 98-59-9(Hazardous Substances Data)
Tosyl chloride Specification
Tosyl chloride , its cas register number is 98-59-9. It also can be called 4-Toluene sulfochloride ; 4-Methylbenzenesulfonyl chloride ; p-Toluenesulfonyl chloride ; Toluene-4-sulfonyl chloride ; 4-Toluolsulfonyl chloride ; and p-Tosyl Chloride .
Related Products
- Tosyl azide
- Tosyl chloride
- Tosyl cyanide
- Tosyl isocyanate
- Tosyl-L-Asparagine
- Tosyl-l-phenylalanylchloromethyl ketone
- Tosylmethyl isocyanide
- Tosylphenylalanyl chloromethyl ketone
- 98600-34-1
- 98601-03-7
- 98-60-2
- 98612-60-3
- 98612-93-2
- 98-61-3
- 98614-76-7
- 98618-52-1
- 98619-07-9
- 986-19-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View