-
Name
p-Anisidine
- EINECS 203-254-2
- CAS No. 104-94-9
- Article Data716
- CAS DataBase
- Density 1.064 g/cm3
- Solubility Soluble in hot water
- Melting Point 56-59 °C(lit.)
- Formula C7H9NO
- Boiling Point 246 °C at 760 mmHg
- Molecular Weight 123.155
- Flash Point 104.5 °C
- Transport Information UN 2811 6.1/PG 3
- Appearance white, fused crystalline solid, characteristic amine odor
- Safety 53-28-36/37-45-61-28A
- Risk Codes 45-26/27/28-33-50
-
Molecular Structure
-
Hazard Symbols
 T+;
T+;  N;
N;  Xi
Xi
- Synonyms 4-Methoxyaniline;4-Methoxy-1-aminobenzene;4-Aminoanisole;1-Amino-4-methoxybenzene;p-Methoxyphenylamine;Benzenamine, 4-methoxy -;4-Anisidine;p-Methoxyaniline;p-Dianisidine;p-Anisylamine;p-Aminoanisole;CCRIS 917;Benzenamine, 4-methoxy-;Anisole, p-amino-;Aniline, p-methoxy-;Aniline, 4-methoxy-;AI3-02392;4-Methoxybenzeneamine;4-Methoxybenzenamine;
- PSA 35.25000
- LogP 1.85860
Synthetic route

| Conditions | Yield |
|---|---|
| With carbon monoxide; water; [Ru(cyclo-octa-1,5-diene)(pyridine)4][BPh4]2 In tetrahydrofuran at 170℃; for 20h; | 100% |
| With hydrogen In ethyl acetate at 20℃; under 7600.51 Torr; for 4h; chemoselective reaction; | 100% |
| With hydrogen In methanol at 20℃; for 2h; | 100% |

| Conditions | Yield |
|---|---|
| With ammonium bromide; ethylenediamine at 70℃; for 5h; Microwave irradiation; | 100% |
| With ammonium bromide; ethylenediamine at 70℃; for 5h; Microwave irradiation; Inert atmosphere; neat (no solvent); | 99% |
| Stage #1: 4-methoxyacetanilide With Schwartz's reagent In tetrahydrofuran at 20℃; for 0.0333333h; Inert atmosphere; Stage #2: With water In tetrahydrofuran Inert atmosphere; chemoselective reaction; | 88% |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; copper(l) iodide; N,N-dimethylethylenediamine In dimethyl sulfoxide at 130℃; for 24h; Reagent/catalyst; Sealed tube; Inert atmosphere; | 99% |
| With copper(l) iodide; 2-carboxyquinoline N-oxide; potassium carbonate; ammonium hydroxide In dimethyl sulfoxide at 80℃; for 23h; Inert atmosphere; | 97% |
| With ammonium hydroxide In water at 20℃; for 9h; Solvent; Reagent/catalyst; Temperature; Green chemistry; | 96% |

| Conditions | Yield |
|---|---|
| With iron(III) oxide; hydrazine hydrate In water at 120℃; for 1.5h; Inert atmosphere; | 99% |
| With iron(III)-acetylacetonate; hydrazine hydrate In methanol at 150℃; for 0.0833333h; Microwave irradiation; chemoselective reaction; | 98% |
| With hydrogen; MCM-silylamine Pd(II) In methanol at 20℃; for 1.5h; Reduction; | 97% |

| Conditions | Yield |
|---|---|
| at 230℃; under 10 Torr; for 0.0833333h; Product distribution; pyrolysis without solvent, isolated as hydrochloride; | A 99% B n/a |
-
-
92851-13-3
benzyl 4-(methoxy)phenylcarbamate

-
-
104-94-9
4-methoxy-aniline

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; silica gel; cyclohexene In methanol at 120℃; for 0.333333h; Flow reactor; | 99% |
| With methanol; sodium tetrahydroborate; nickel(II) chloride hexahydrate at 20℃; for 0.25h; chemoselective reaction; | 88% |
| With boron trifluoride diethyl etherate; ethanethiol for 32h; Ambient temperature; | 81% |
| With ammonium formate; palladium on activated charcoal In isopropyl alcohol for 0.05h; Irradiation; |
-
-
18437-68-8
tert-butyl N-(4-methoxyphenyl)carbamate

-
-
104-94-9
4-methoxy-aniline

| Conditions | Yield |
|---|---|
| With water at 100℃; for 6h; Inert atmosphere; | 99% |
| In 2,2,2-trifluoroethanol at 150℃; for 2h; microwave irradiation; | 98% |
| With kaolin In dichloromethane for 1.5h; deacylation; Heating; | 97% |
-
-
10507-69-4
4-methoxybenzohydroxamic acid

-
-
104-94-9
4-methoxy-aniline

| Conditions | Yield |
|---|---|
| With potassium carbonate In dimethyl sulfoxide at 90℃; for 2h; Lossen rearrangement; | 99% |
| With potassium carbonate In dimethyl sulfoxide at 90℃; for 2h; Lossen Rearrangement; | 99% |
| Stage #1: 4-methoxybenzohydroxamic acid With acetic anhydride; potassium carbonate In dimethyl sulfoxide at 50℃; for 2h; Lossen Rearrangement; Stage #2: With hydrogenchloride In water; dimethyl sulfoxide at 0℃; | 5% |
| With sodium hydroxide In dimethyl sulfoxide at 80℃; for 1.5h; Lossen rearrangement; |
-
-
83507-70-4
diethyl 2-(((4-methoxyphenyl)amino)methylene)malonate

-
-
104-94-9
4-methoxy-aniline

| Conditions | Yield |
|---|---|
| With ethylenediamine In ethanol at 20℃; for 0.333333h; | 99% |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; potassium phosphate In dimethyl sulfoxide at 80℃; UV-irradiation; | 98% |
| With tris(dibenzylideneacetone)dipalladium (0); lithium hexamethyldisilazane; CyJohnPhos In tetrahydrofuran at 65℃; for 15h; | 95% |
| With copper(ll) sulfate pentahydrate; ammonium hydroxide In PEG1000-DIL; methyl cyclohexane at 60℃; for 8h; | 90% |
-
-
92097-26-2
(4-Methoxy-phenyl)-carbamic acid (E)-4-trimethylsilanyl-but-2-enyl ester

-
-
104-94-9
4-methoxy-aniline

| Conditions | Yield |
|---|---|
| With methanol; tetrakis(triphenylphosphine) palladium(0) In dichloromethane for 0.333333h; Ambient temperature; | 97% |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; copper(l) iodide; N,N-dimethylethylenediamine In dimethyl sulfoxide at 130℃; for 18h; Reagent/catalyst; Time; Sealed tube; Inert atmosphere; | 97% |
| With ammonium hydroxide In water at 20℃; for 9h; Green chemistry; | 97% |
| Stage #1: para-iodoanisole With potassium phosphate; 2,2,2-trifluoroacetamide; N,N`-dimethylethylenediamine; copper(l) iodide In N,N-dimethyl-formamide at 45℃; Stage #2: With methanol In N,N-dimethyl-formamide at 45℃; Further stages.; | 95% |
-
-
965-50-4
N-(4-methoxyphenyl)-3-oxo-3-phenylpropanamide

-
-
95-54-5
1,2-diamino-benzene

-
A
-
104-94-9
4-methoxy-aniline

-
B
-
716-79-0
2-phenyl-1H-benzoimidazole

| Conditions | Yield |
|---|---|
| With 1-n-butyl-3-methylimidazolim bromide In neat (no solvent) at 120℃; for 1.5h; | A 92% B 97% |

-
-
1562-94-3, 21650-70-4, 51437-65-1
4,4'-dimethoxyazoxybenzene

-
-
104-94-9
4-methoxy-aniline

| Conditions | Yield |
|---|---|
| With N-doped TiO2 In methanol at 20℃; for 3h; UV-irradiation; Inert atmosphere; | 95% |
| With 4,4'-di-tert-butylbiphenyl; lithium; nickel dichloride In tetrahydrofuran at 20℃; for 2h; Reduction; deoxygenation; | 57% |
-
-
5437-98-9
4'-methoxyacetoacetanilide

-
-
95-54-5
1,2-diamino-benzene

-
A
-
615-15-6
2-Methyl-1H-benzimidazole

-
B
-
104-94-9
4-methoxy-aniline

| Conditions | Yield |
|---|---|
| With 1-n-butyl-3-methylimidazolim bromide In neat (no solvent) at 120℃; for 1h; | A 95% B 93% |


| Conditions | Yield |
|---|---|
| With bis[(2-methylacetatobenzyl)tri(p-tolyl)phosphonium] hexabromodipalladate(II); potassium carbonate at 90℃; for 0.3h; Stille Cross Coupling; Green chemistry; | 95% |

| Conditions | Yield |
|---|---|
| With 3-azapentane-1,5-diamine at 130℃; for 12h; Sealed tube; | 94% |
| In water at 150℃; Green chemistry; |

| Conditions | Yield |
|---|---|
| With ammonium bromide; ethylenediamine at 80℃; for 5h; Microwave irradiation; | 94% |
| With 3-azapentane-1,5-diamine at 130℃; for 12h; Sealed tube; | 92% |

| Conditions | Yield |
|---|---|
| With aminomethyl polysterene resine formic acid salt; zinc In methanol at 20℃; for 0.333333h; | 93% |
| With potassium hydroxide; nickel-incorporated hexagonal mesoporous aluminophosphate In isopropyl alcohol at 82.84℃; for 1.5h; | 91% |
| With zinc In methanol at 25℃; for 0.216667h; Inert atmosphere; | 90% |
-
-
78-83-1
2-methyl-propan-1-ol

-
-
458-52-6
2-fluoro-4-(methoxy)aniline

-
A
-
71182-60-0
N-isobutyl-N-(4-methoxyphenyl)amine

-
B
-
104-94-9
4-methoxy-aniline

| Conditions | Yield |
|---|---|
| With methanesulfonato(2-dicyclohexylphosphino -2’,6’-di-i-propoxy-1,1‘-biphenyl)(2’-amino-1,1’-biphenyl-2-yl)palladium(II); sodium tert-pentoxide In toluene at 80℃; for 72h; Sealed tube; Inert atmosphere; | A 93% B 2% |


| Conditions | Yield |
|---|---|
| With C25H19BrMnN2O2P; potassium tert-butylate; hydrogen In toluene at 130℃; under 15001.5 Torr; for 48h; | A 90 %Spectr. B 93% |
-
-
635-46-1
1,2,3,4-tetrahydroisoquinoline

-
-
100-17-4
para-methoxynitrobenzene

-
A
-
91-22-5
quinoline

-
B
-
104-94-9
4-methoxy-aniline

| Conditions | Yield |
|---|---|
| With nickel-nitrogen-doped carbon framework In water at 145℃; for 18h; Inert atmosphere; Sealed tube; Green chemistry; | A 92% B 93% |

-
-
58960-03-5
7-methyl-1,2,3,4-tetrahydroquinoline

-
-
100-17-4
para-methoxynitrobenzene

-
A
-
612-60-2
7-methylquinoline

-
B
-
104-94-9
4-methoxy-aniline

| Conditions | Yield |
|---|---|
| With nickel-nitrogen-doped carbon framework In water at 145℃; for 18h; Inert atmosphere; Sealed tube; Green chemistry; | A 92% B 92% |

-
-
110228-48-3
4'-methoxybiphenyl-2-sulphenanilide

-
A
-
5051-19-4
2,7-dimethoxyphenazine

-
B
-
19813-97-9
1-phenyl-2-[(2-phenylphenyl)disulfanyl]benzene

-
C
-
104-94-9
4-methoxy-aniline

-
D
-
501-58-6, 21650-55-5, 82570-64-7
(E)-1,2-bis(4-methoxyphenyl)diazene

| Conditions | Yield |
|---|---|
| In benzene at 150℃; for 40h; Further byproducts given; | A 26% B 91% C 17% D 12% |
| In benzene at 85℃; for 720h; Further byproducts given; | A 12% B 81% C 29% D 12% |


| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide; CYANAMID; bis-[(trifluoroacetoxy)iodo]benzene In acetonitrile at 20℃; for 1h; chemoselective reaction; | 91% |
| With copper(I) oxide; ammonium hydroxide; oxygen; sodium hydroxide In water at 25℃; for 15h; | 88% |
| With sodium hydroxide; hydroxylamine-O-sulfonic acid In acetonitrile at 20℃; for 16h; | 86% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide In water at 200℃; for 2h; Autoclave; | 91% |
| With copper(ll) sulfate pentahydrate; potassium phosphate tribasic heptahydrate; water; Sucrose In water at 90℃; for 15h; Catalytic behavior; Reagent/catalyst; Solvent; Green chemistry; | 85% |
-
-
371779-94-1
N,N-bis(1,1-dioxo-1,2-benzisothiazol-3-yl)-4-methoxyaniline

-
-
104-94-9
4-methoxy-aniline

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In tetrahydrofuran for 12h; | 90% |

-
-
109-77-3
malononitrile

-
A
-
104-94-9
4-methoxy-aniline

-
B
-
137100-26-6
1-chloro-6-methoxy-2-[β,β-dicyano]-3,4-dihydronaphthalene

| Conditions | Yield |
|---|---|
| With sodium dodecyl-sulfate In water at 25 - 30℃; for 0.166667h; | A n/a B 90% |

-
-
171364-79-7
2-(4-methoxyphenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane

-
-
104-94-9
4-methoxy-aniline

| Conditions | Yield |
|---|---|
| With water; sodium hydroxide; hydroxylamine-O-sulfonic acid In acetonitrile at 20℃; for 16h; | 90% |
| With O-Methylhydroxylamin; n-butyllithium In tetrahydrofuran; hexane at -78 - 60℃; for 24h; Inert atmosphere; Cooling with acetone-dry ice; stereospecific reaction; | 87% |

| Conditions | Yield |
|---|---|
| With hydrogen In toluene at 160℃; under 45004.5 Torr; for 15h; Catalytic behavior; Autoclave; | A 90% B 82% |
| With potassium tert-butylate; hydrogen; [Ru(PtBuNNHBn)H(CO)Cl] In tetrahydrofuran at 19 - 24℃; under 7500.75 Torr; for 68h; | A 92 %Chromat. B 92 %Chromat. |

| Conditions | Yield |
|---|---|
| With sodium sulfate In benzene for 0.5h; Ambient temperature; | 100% |
| In methanol at 20℃; for 24h; | 100% |
| In methanol at 20℃; for 24h; | 100% |

| Conditions | Yield |
|---|---|
| at 160℃; for 6h; Inert atmosphere; Neat (no solvent); | 100% |
| With palladium 10% on activated carbon; sodium t-butanolate; CyJohnPhos In tert-Amyl alcohol at 110℃; Buchwald-Hartwig amination; Inert atmosphere; | 73% |
| With sodium dispersion In tetrahydrofuran at 50℃; for 1h; | 65.6% |
| With zinc(II) chloride at 220 - 230℃; |

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; Inert atmosphere; | 100% |
| With silver trifluoromethanesulfonate at 60℃; for 0.0166667h; neat (no solvent); | 99% |
| With cadmium(II) oxide at 80℃; for 0.0833333h; Neat (no solvent); Microwave irradiation; | 98% |
-
-
104-88-1
4-chlorobenzaldehyde

-
-
104-94-9
4-methoxy-aniline

-
-
1749-03-7
N-(4-chlorobenzylidene)-4-methoxyaniline

| Conditions | Yield |
|---|---|
| for 6h; Ambient temperature; | 100% |
| In ethanol for 1h; Sonication; | 100% |
| at 20℃; for 14h; Molecular sieve; | 99% |
-
-
555-16-8
4-nitrobenzaldehdye

-
-
104-94-9
4-methoxy-aniline

-
-
5455-87-8
4-methoxy-N-(4-nitrobenzylidene)aniline

| Conditions | Yield |
|---|---|
| at 50℃; for 24h; | 100% |
| for 6h; Molecular sieve; Reflux; | 100% |
| In ethanol for 3h; Reflux; | 95% |

| Conditions | Yield |
|---|---|
| In toluene for 1h; Ambient temperature; | 100% |
| In ethanol for 1h; Sonication; | 100% |
| In dichloromethane at 20℃; Molecular sieve; | 99% |
-
-
123-08-0
4-hydroxy-benzaldehyde

-
-
104-94-9
4-methoxy-aniline

-
-
3230-50-0
N-(4-hydroxylbenzylidene)-4-methoxyaniline

| Conditions | Yield |
|---|---|
| for 6h; Ambient temperature; | 100% |
| With sulfuric acid In neat (no solvent) Microwave irradiation; Sealed tube; Green chemistry; | 93% |
| In ethanol for 5h; Reflux; | 88% |
-
-
123-11-5
4-methoxy-benzaldehyde

-
-
104-94-9
4-methoxy-aniline

-
-
1749-08-2
N-(4-methoxy benzylidene)-4-methoxyaniline

| Conditions | Yield |
|---|---|
| In ethanol for 1h; Sonication; | 100% |
| With magnesium sulfate In dichloromethane at 20℃; for 72h; | 99% |
| In ethyl 2-hydroxypropionate; water at 20℃; for 0.05h; | 93% |

| Conditions | Yield |
|---|---|
| Stage #1: carbon disulfide; 4-methoxy-aniline With triethylamine In ethanol at 20℃; Stage #2: With dmap; di-tert-butyl dicarbonate In ethanol at 20℃; for 0.25h; Further stages.; | 100% |
| Stage #1: carbon disulfide; 4-methoxy-aniline With triethylamine In ethanol at 20℃; for 1h; Stage #2: With dmap; di-tert-butyl dicarbonate at 0 - 20℃; for 4h; | 96% |
| Stage #1: carbon disulfide; 4-methoxy-aniline With triethylamine In methanol at 0 - 5℃; Stage #2: With bis(trichloromethyl) carbonate In chloroform at 0℃; for 4h; Further stages.; | 95% |

| Conditions | Yield |
|---|---|
| In toluene Reflux; | 100% |
| With sodium formate at 20℃; for 3h; Neat (no solvent); | 99% |
| With TiO2-SO4(2-) In acetonitrile at 20℃; for 4h; | 99% |
-
-
104-94-9
4-methoxy-aniline

-
-
121-33-5
vanillin

-
-
24033-07-6
4-methoxy-N-(4-hydroxy-3-methoxybenzylidene)aniline

| Conditions | Yield |
|---|---|
| for 2h; Ambient temperature; | 100% |
| In ethanol for 3h; Reflux; | 99.2% |
| sodium hydrogen sulfate; silica gel at 60 - 62℃; for 0.0222222h; microwave irradiation; | 98% |

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 0 - 20℃; for 15h; | 100% |
| With sodium hydroxide at 120℃; |
-
-
104-94-9
4-methoxy-aniline

-
-
556-61-6
methyl thioisocyanate

-
-
20333-73-7
1-(4-methoxyphenyl)-3-methyl-thiourea

| Conditions | Yield |
|---|---|
| at 20℃; for 24h; Addition; solid-phase reaction; | 100% |
| With ethanol | |
| In ethanol for 1h; Heating; | |
| In ethanol Reflux; |
-
-
104-94-9
4-methoxy-aniline

-
-
292638-85-8
acrylic acid methyl ester

-
-
42313-52-0
methyl 3-(4-methoxyphenylamino)propanoate

| Conditions | Yield |
|---|---|
| With silica-supported aluminum chloride at 60℃; for 1.5h; Michael addition; Neat (no solvent); | 100% |
| In water at 50℃; for 24h; Michael condensation; | 94% |
| With lithium tetrafluoroborate at 70℃; for 0.166667h; Michael condensation; Neat (no solvent); | 93% |
-
-
104-94-9
4-methoxy-aniline

-
-
79-04-9
chloroacetyl chloride

-
-
22303-36-2
N-(4-methoxyphenyl)-2-chloroacetamide

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 0℃; for 0.166667h; | 100% |
| In tetrahydrofuran at 20℃; for 18h; | 100% |
| With triethylamine In dichloromethane at 0 - 25℃; | 99.4% |
-
-
104-94-9
4-methoxy-aniline

-
-
104-55-2
3-phenyl-propenal

-
-
15286-52-9, 22835-33-2, 80542-40-1, 88315-63-3, 101535-86-8, 123525-44-0
N-(4-Methoxyphenyl)-3-phenylpropenaldimine

| Conditions | Yield |
|---|---|
| piperidine In ethanol for 5h; Heating; | 100% |
| With magnesium sulfate In dichloromethane at 20℃; | 100% |
| In ethyl 2-hydroxypropionate; water at 20℃; for 0.025h; | 96% |
-
-
104-94-9
4-methoxy-aniline

-
-
1885-14-9
phenyl chloroformate

-
-
20950-96-3
(4-methoxyphenyl)carbamic acid phenyl ester

| Conditions | Yield |
|---|---|
| With pyridine In ethyl acetate at 20℃; for 1h; | 100% |
| With sodium hydrogencarbonate In tetrahydrofuran; water at 0 - 20℃; for 0.0833333h; | 97% |
| With sodium carbonate In tetrahydrofuran; water; ethyl acetate at 0 - 20℃; | 92% |
-
-
104-94-9
4-methoxy-aniline

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
1150-26-1
N-(4-Methoxy-phenyl)-4-methyl-benzenesulfonamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 4℃; | 100% |
| With pyridine for 16h; Reflux; | 97% |
| With dendritic fibrous nanosilica KCC-1 3-aminopropyl-functionalized supported on Fe3O4 magnetic nanocatalyst In water at 20℃; for 0.5h; Solvent; Temperature; Reagent/catalyst; Time; Green chemistry; | 97% |
-
-
104-94-9
4-methoxy-aniline

-
-
97-00-7
1-chloro-2,4-dinitro-benzene

-
-
967-35-1
N-(4-methoxyphenyl)-2,4-dinitrobenzenamine

| Conditions | Yield |
|---|---|
| With sodium carbonate In ethanol Heating; | 100% |
| In benzene at 40℃; Rate constant; Mechanism; other solvent; | |
| With tetrabutyl-ammonium chloride In benzene at 30℃; Rate constant; different substrate concentrations; |
-
-
104-94-9
4-methoxy-aniline

-
-
407-25-0
trifluoroacetic anhydride

-
-
332-34-3
2,2,2-trifluoro-N-(4-methoxy-phenyl)-acetamide

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 20℃; | 100% |
| With triethylamine In tetrahydrofuran at 0 - 20℃; for 0.75h; | 100% |
| at 20℃; for 1h; | 98.3% |
-
-
104-94-9
4-methoxy-aniline

-
-
121-90-4
m-nitrobenzoic acid chloride

-
-
101971-72-6
N-(4'-methoxyphenyl)-3-nitrobenzamide

| Conditions | Yield |
|---|---|
| In pyridine Heating; | 100% |
| With pyridine Reflux; | 85% |
| With triethylamine In acetone at 20℃; | 47% |

| Conditions | Yield |
|---|---|
| With chloro(η5-pentamethylcyclopentadienyl)(L-prolinato)iridium(III) In toluene at 95℃; for 24h; Inert atmosphere; Sealed tube; | 100% |
| With potassium tert-butylate; copper diacetate In 1,4-dioxane at 130℃; for 48h; | 99% |
| With potassium tert-butylate; copper diacetate In 1,4-dioxane at 130℃; for 48h; Inert atmosphere; | 99% |
-
-
104-94-9
4-methoxy-aniline

-
-
87-13-8
diethyl 2-ethoxymethylenemalonate

-
-
83507-70-4
diethyl 2-(((4-methoxyphenyl)amino)methylene)malonate

| Conditions | Yield |
|---|---|
| at 14 - 95℃; for 2.91667h; | 100% |
| In ethanol for 4h; Heating / reflux; | 100% |
| at 100 - 125℃; for 18.5h; | 100% |

| Conditions | Yield |
|---|---|
| With tert.-butylnitrite; trimethylsilylazide In acetonitrile at 0 - 20℃; for 1h; | 100% |
| With 2-azido-1,3-dimethyl-4,5-dihydro-1H-imidazol-3-ium hexafluorophosphate (V); triethylamine In dichloromethane at 20℃; for 0.166667h; Solvent; Reagent/catalyst; Temperature; Inert atmosphere; | 100% |
| Stage #1: 4-methoxy-aniline With hydrogenchloride; sodium nitrite In water for 0.5h; Cooling with ice; Stage #2: With sodium azide In water at 20℃; for 2h; Cooling with ice; | 100% |
-
-
126-81-8
dimedone

-
-
104-94-9
4-methoxy-aniline

-
-
24706-48-7
3-[(4-methoxyphenyl)amino]-5,5-dimethylcyclohex-2-en-1-one

| Conditions | Yield |
|---|---|
| at 20℃; for 0.5h; Solid phase reaction; condensation; | 100% |
| With silica-supported phosphorous pentoxide at 80℃; for 0.05h; Neat (no solvent); chemoselective reaction; | 97% |
| With silica sulfuric acid In acetonitrile for 0.0166667h; Microwave irradiation; | 97% |

| Conditions | Yield |
|---|---|
| With potassium hydrogensulfate; water; sodium nitrite Diazotization; coupling; microwave irradiation; | 100% |
| Stage #1: 4-methoxy-aniline With ferric hydrogen sulphate; silica gel; sodium nitrite In water Green chemistry; Stage #2: β-naphthol In water at 20℃; for 0.05h; Green chemistry; regioselective reaction; | 97% |
| Stage #1: 4-methoxy-aniline With water; sodium nitrite In neat (no solvent) at 20℃; Stage #2: β-naphthol In neat (no solvent) at 20℃; for 0.25h; | 94% |
-
-
1121-60-4
pyridine-2-carbaldehyde

-
-
104-94-9
4-methoxy-aniline

-
-
26930-67-6, 42910-70-3
(E)-N-(4-methoxyphenyl)-1-(pyridin-2-yl)methanimine

| Conditions | Yield |
|---|---|
| In toluene at 20℃; for 24h; | 100% |
| With magnesium sulfate In benzene for 20h; Ambient temperature; | 98% |
| With magnesium sulfate In ethanol at 25℃; for 16h; | 91% |
-
-
620-02-0
5-Methylfurfural

-
-
104-94-9
4-methoxy-aniline

-
-
95124-23-5
N-(4-methoxyphenyl)-1-(5-methylfuran-2-yl)methanimine

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In diethyl ether at 20℃; Molecular sieve; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| 1.) water, reflux, 24 h 2.) without water, 60 deg C, 30 min; | 100% |
p-Anisidine Specification
The CAS registry number of p-Anisidine is 104-94-9. Its EINECS registry number is 203-254-2. The IUPAC name is 4-methoxyaniline. In addition, the molecular formula is C7H9NO and the molecular weight is 123.15. It is also called 1-amino-4-methoxybenzene. What's more, it is a grey-brown solid and belongs to the classes of Intermediates of Dyes and Pigments; Anilines (Building Blocks for Liquid Crystals); Building Blocks for Liquid Crystals; Functional Materials. Besides, it should be stored in sealed container, and put in a cool and dry place. The storage place must stay away from oxidant, the fire and heat source
Physical properties about this chemical are: (1)ACD/LogP: 0.94; (2)ACD/LogD (pH 5.5): 0.758; (3)ACD/LogD (pH 7.4): 0.933; (4)ACD/BCF (pH 5.5): 2.011; (5)ACD/BCF (pH 7.4): 3.01; (6)ACD/KOC (pH 5.5): 51.073; (7)ACD/KOC (pH 7.4): 76.456; (8)#H bond acceptors: 2; (9)#H bond donors: 2; (10)#Freely Rotating Bonds: 2; (11)Polar Surface Area: 35.25 Å2; (12)Index of Refraction: 1.555; (13)Molar Refractivity: 37.167 cm3; (14)Molar Volume: 115.716 cm3; (15)Polarizability: 14.734 ×10-24cm3; (16)Surface Tension: 39.327 dyne/cm; (17)Density: 1.064 g/cm3; (18)Flash Point: 104.537 °C; (19)Enthalpy of Vaporization: 48.31 kJ/mol; (20)Boiling Point: 245.999 °C at 760 mmHg; (21)Vapour Pressure: 0.028 mmHg at 25°C.
Preparation of p-Anisidine: it can be prepared by p-nitroanisole with sodium sulfide. Add sodium sulfide into the reactor and heat it to 90 °C with stirring. Then add p-nitroanisole into the reactor in 4 hours. The reaction temperature should be controlled at 90-95 °C. After adding the p-nitroanisole, heat the mixture to 100 °C with stirring for 0.5 hour. Then heat it to 110-111 °C with stirring for 2 hours. And then after a series of separation and vacuum distillation you can get the desired product.
Uses of p-Anisidine: it is often used for biochemical research and organic synthesis. And it can be used as pharmaceutical intermediates. In addition, it can react with vinylbenzene to get 1-methoxy-4-trans-styryl-benzene. This reaction will need reagent t-BuONO, monochloroacetic acid, catalyst Pd(dba)2 and solvent acetic acid. The reaction time is 0.5 hour at reaction temperature of 50 °C. The yield is about 67%.
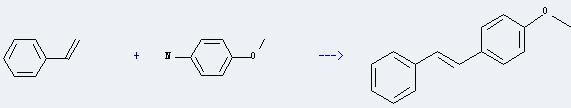
When you are using this chemical, please be cautious about it as the following:
This chemical is very toxic by inhalation, in contact with skin and if swallowed. And it has danger of cumulative effects and may cause cancer. Moreover, it is very toxic to aquatic organisms. If heated strongly, it may release very toxic fumes of nitrogen oxides. You should avoid exposure - obtain special instruction before use. When you are using it, wear suitable protective clothing and gloves. If contact it with skin, wash it immediately with plenty of ... (to be specified by the manufacturer). In case of accident or if you feel unwell, seek medical advice immediately (show label where possible). In addition, you should avoid release to the environment and you can refer to special instructions safety data sheet.
You can still convert the following datas into molecular structure:
(1)SMILES: COc1ccc(cc1)N
(2)InChI: InChI=1/C7H9NO/c1-9-7-4-2-6(8)3-5-7/h2-5H,8H2,1H3
(3)InChIKey: BHAAPTBBJKJZER-UHFFFAOYAR
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 806mg/kg (806mg/kg) | Journal of Medicinal Chemistry. Vol. 17, Pg. 900, 1974. | |
| mouse | LD50 | oral | 1410mg/kg (1410mg/kg) | SENSE ORGANS AND SPECIAL SENSES: OTHER: EYE BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BEHAVIORAL: ATAXIA | Acute Toxicity Data. Journal of the American College of Toxicology, Part B. Vol. 1, Pg. 184, 1992. |
| rabbit | LD50 | oral | 2900mg/kg (2900mg/kg) | KIDNEY, URETER, AND BLADDER: OTHER CHANGES BLOOD: OTHER CHANGES BLOOD: NORMOCYTIC ANEMIA | IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. Vol. 27, Pg. 63, 1982. |
| rat | LD50 | intraperitoneal | 1400mg/kg (1400mg/kg) | Archiv fuer Gewerbepathologie und Gewerbehygiene. Vol. 15, Pg. 447, 1957. | |
| rat | LD50 | oral | 1320mg/kg (1320mg/kg) | Trudy Leningradskogo Sanitarno-Gigienicheskogo Meditsinskogo Instituta. Vol. 128, Pg. 14, 1979. | |
| rat | LD50 | skin | 3200mg/kg (3200mg/kg) | Archiv fuer Gewerbepathologie und Gewerbehygiene. Vol. 15, Pg. 447, 1957. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View