Dayang Chem (Hangzhou) Co.,Ltd.
DayangChem exported this product to many countries and regions at best price. If you are looking for the material's manufacturer or supplier in China, DayangChem is your best choice. Pls contact with us freely for getting detailed product spe
Cas:218600-53-4
Min.Order:1 Kilogram
FOB Price: $3.0
Type:Lab/Research institutions
inquiryXi'an Xszo Chem Co., Ltd.
1. Factory price and high quality must be guaranteed, base on 8 years of production and R&D experience2. Free samples will be provided,ensure specifications and quality are right for customer3. Customers will receive the most professional technical s
Cas:218600-53-4
Min.Order:1 Gram
FOB Price: $0.1
Type:Manufacturers
inquiryShanghai Upbio Tech Co.,Ltd
1.In No Less 10 years exporting experience. you can 100% received goods 2.Lower Price with higher quality 3,Free sample 4,We are sincerely responsible for the "product quality" and "After Service" Upbio is Specializ
Cas:218600-53-4
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryQingdao Beluga Import and Export Co., LTD
Beluga chemical professional supply Bardoxolone Methyl CAS 872-36-6 1. Beluga Chemical has a professional RESEARCH and development team and strong technical force to ensure technical support and research capabilities. 2. Made in China and exporte
Cas:218600-53-4
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryHubei CuiRan Biotechnology Co., Ltd
Hubei CuiRan Biotechnology Co., Ltd is a leading company in the research, development, manufacture and marketing of High Quality Phytochemicals and Extracts(especially Active Ingredients from Traditional Chinese Medicine,Traditional Chinese Medicine)
Cas:218600-53-4
Min.Order:10 Milligram
Negotiable
Type:Lab/Research institutions
inquiryHebei Quanhe Biotechnology Co. LTD
1. Timely and efficient service to ensure communication with customers 2. Produce products of different specifications and sizes according to your requirements. 3. Quality procedures and standards recognized by SGS. Advanced plant equipment ensures s
Cas:218600-53-4
Min.Order:1 Kilogram
FOB Price: $3.0 / 5.0
Type:Lab/Research institutions
inquiryHenan Wentao Chemical Product Co., Ltd.
Henan Wentao Chemical Product Co.,Ltd is Located in Zhengzhou High-tech Development Zone with import and export license, We passed ISO 9001:2008 as well, Henan Wentao has developed more than 1000 compounds, which are widely used in the fields of prod
Cas:218600-53-4
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryTaizhou Crene Biotechnology co.ltd
Our company provides one-stop services of research - development - production for a variety of special prouducts. Not only do we make effective use of our strong technological strength, but also establish of cooperative relations with several well-
Cas:218600-53-4
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryZibo Hangyu Biotechnology Development Co., Ltd
Zibo Hangyu Biotechnology Development Co., Ltd is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemi
Cas:218600-53-4
Min.Order:10 Gram
FOB Price: $100.0
Type:Lab/Research institutions
inquiryHangzhou Lingrui Chemical Co.,Ltd.
advantage: 1. The best price, satisfactory quality; 2. customers have the right to choose the delivery of parcels (EMS, DHL, FedEx, UPS); 3. customers have the right to choose from the recent effective packaging methods of their products packaging
Cas:218600-53-4
Min.Order:1 Gram
Negotiable
Type:Other
inquiryHANGZHOU YUNUO CHEMICAL CO.,LTD
Superior quality, moderate price & quick delivery. Appearance:White to Off-White Solid Storage:Stored in cool, dry and ventilation place; Away from fire and heat Package:1kg/bag, 1kg/drum or 25kg/drum or as per your request. Application:CDDO me
Cas:218600-53-4
Min.Order:1 Kilogram
Negotiable
Type:Trading Company
inquiryXiamen Jenny Chemical Technology Co., Ltd.
GMP standard, high purity, competitive price, in stock 1. Quick Response: within 6 hours after receiving your email. 2. Quality Guarantee: All products are strictly tested by our QC, confirmed by QA, and approved by a third-party lab in China, USA,
Cas:218600-53-4
Min.Order:1 Milligram
Negotiable
Type:Trading Company
inquiryHunan chemfish Pharmaceutical co.,Ltd
Appearance:95%+ Package:R&D,Pilot run Transportation:per client require Port:Express ,Air, Sea
Cas:218600-53-4
Min.Order:0
Negotiable
Type:Manufacturers
inquirySAGECHEM LIMITED
SAGECHEM is a chemical R&D, manufacturing and distribution company in China since 2009, including pharmaceutical intermediates, agrochemical, dyestuff intermediates, organosilicone, API and etc. We also offer a full range of services in custom synthe
Cas:218600-53-4
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryBluecrystal chem-union
We are a Union of chemistry in China, consists of chemists,engineers, laboratories,factories in China. We organize surplus capacity of R&D and production as well as custom synthesis for chemical products and chemical business project. We are supp
Win-Win chemical Co.Ltd
Stock products, own laboratory Package:Grams, Kilograms Application:For R&D Transportation:According to customer request Port:Shanghai
Cas:218600-53-4
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryHenan Allgreen Chemical Co.,Ltd
high quality Storage:Sealed, dry, microtherm , avoid light and smell. Package:According to the demand of customer Application:Organic synthesis Transportation:by air or by sea
BOC Sciences
BOC Sciences is committed to supplying cost-effective products and services. We provide Bardoxolone methyl (Cas:218600-53-4). Bardoxolone methyl (also known as “RTA 402” and “CDDO-methyl ester”) is an orally-available first-
Hangzhou J&H Chemical Co., Ltd.
J&H CHEM is one of China's leading providers of integrated fine chemical services including offering, research and development, Custom manufacturing business, as well as other Value-added customer services, for diversified range products of chemicals
Henan Tianfu Chemical Co., Ltd.
1.Our services:A.Supply sampleB.The packing also can be according the customers` requirmentC.Any inquiries will be replied within 24 hoursD.we provide Commerical Invoice, Packing List, Bill of loading, COA , Health certificate and Origin certificate.
Cas:218600-53-4
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryAntimex Chemical Limied
Ansciep Chemical is a professional enterprise manufacturing and distributing fine chemicals and speciality chemicals. We have been dedicated to heterocycle compounds and phenyl rings for tens of years. This is our mature product for export. Our quali
Guangdong Juda Chemical Industrial Co.,Limited
Factory supply high purity low priceAppearance:solid or liquid Storage:sealed in cool and dry place Package:As customer's requested Application:Pharma Intermediate Transportation:by courier/air/sea Port:Any port in China
Wuhan Circle Star Chem-medical Technology co.,Ltd.
good quality, competitive price, thoughtful after sale serviceAppearance:white powder Storage:Keep it in dry,shady and cool place Package:25kg,50kg,180kg,200kg,250kg,1000kg,customization Application:Pharma;Industry;Agricultural;chemical reaserch Tran
Cas:218600-53-4
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryHangzhou Fandachem Co.,Ltd
Oleana-1,9(11)-dien-28-oicacid, 2-cyano-3,12-dioxo-, methyl esterAppearance:white crystalline powder Storage:Store in dry, dark and ventilated place Package:25KG drum Application:pharmaceutical intermediate Transportation:by air, by sea, by express
Cas:218600-53-4
Min.Order:0
Negotiable
Type:Other
inquiryHenan Kanbei Chemical Co.,LTD
factory?direct?saleAppearance:White powder Storage:Sealed and preserved Package:200/Kilograms Application:healing drugs Transportation:By sea Port:Shanghai/tianjin
Shanghai Send Pharmaceutical Technology Co., Ltd.
low price high quality high purity good sevice in stock Storage:Preserve In Well-Closed, Light-Resistant and Tight Containers. Store In Cool & Dry Place Package:1g,5g,10g...1kg,5kg...more Application:R&D Transportation:shipping,land,air Port:Sh
Cas:218600-53-4
Min.Order:100 Gram
Negotiable
Type:Lab/Research institutions
inquiryHunan Russell Chemicals Technology Co.,Ltd
high purity lowest priceAppearance:solid or liquid Storage:in sealed air resistant place Package:Foil bag; Drum; Plastic bottle Application:Pharma;Industry;Agricultural Transportation:by sea or air Port:any port in China
Cas:218600-53-4
Min.Order:0
Negotiable
Type:Trading Company
inquiryShanghai Yuanye Bio-Technology Co., Ltd.
good quality, competitive price, thoughtful after sale serviceAppearance:white powder Storage:Keep it in dry,shady and cool place Package:10mg Application:Pharma;Industry;Agricultural;chemical reaserch Transportation:by express or by sea Port:Any por
Chemlyte Solutions
Stock products, own laboratory Package:Grams, Kilograms Application:For R&D Transportation:According to customer request Port:Shanghai
Cas:218600-53-4
Min.Order:0
Negotiable
Type:Other
inquiryShanghai Chinqesen Biotechnology Co., Ltd.
Good Quality Package:1kg/bag Application:Medical or chemical Transportation:Air/Train/Sea Port:Shenzhen
Synthetic route
-
-
1428550-98-4
2-bromo-3,12-dioxo-oleanane-1,9 (11)-diene-28-carboxylic acid methyl ester

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| With potassium iodide In N,N-dimethyl-formamide at 120℃; for 30h; | 83.6% |
| With potassium iodide In N,N-dimethyl-formamide at 120℃; | 73% |
| With potassium iodide In N,N-dimethyl-formamide at 120℃; | 73% |
| With potassium iodide In N,N-dimethyl-formamide at 120℃; for 24h; Temperature; Time; Reagent/catalyst; Inert atmosphere; | 66% |

| Conditions | Yield |
|---|---|
| Stage #1: bardoxolone With oxalyl dichloride; N,N-dimethyl-formamide In dichloromethane at 20℃; for 1.16667h; Cooling with ice; Stage #2: methanol In dichloromethane for 1h; | 96% |

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In benzene for 0.5h; Reflux; Inert atmosphere; | 87% |
| Stage #1: methyl 2-cyano-3,12-dioxoolean-9(11)-en-28-oate With Phenylselenyl chloride In ethyl acetate Stage #2: With dihydrogen peroxide In tetrahydrofuran; water | 40% |
| With Phenylselenyl chloride; dihydrogen peroxide 1.) ethyl acetate, 2.) THF; Yield given; Multistep reaction; |

| Conditions | Yield |
|---|---|
| With potassium iodide In N,N-dimethyl-formamide at 120℃; for 16h; Inert atmosphere; | 89% |
-
-
305818-40-0
1,2-dihydro-2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid methyl ester

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In benzene for 0.5h; Heating; | 92% |

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| With porcine liver esterase; γ-glutamyl transpeptidase In aq. buffer at 37℃; for 8h; pH=7.4; Enzymatic reaction; | 88% |
-
-
508-02-1
Oleanolic acid

-
-
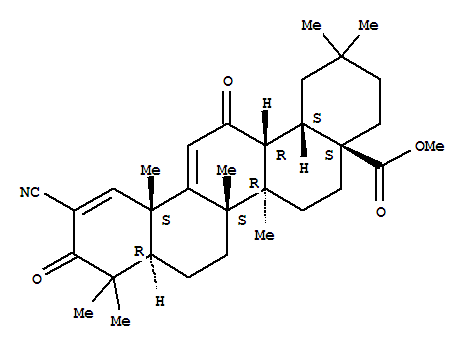
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: potassium carbonate / N,N-dimethyl-formamide / 24 h / 0 - 20 °C / Inert atmosphere 2.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione; fluorobenzene / dimethyl sulfoxide / 24 h / 85 °C / Inert atmosphere 3.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 24 h / 0 - 20 °C / Inert atmosphere 3.2: 24 h / 35 °C / Inert atmosphere 4.1: potassium iodide / N,N-dimethyl-formamide / 24 h / 120 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 4 steps 1.1: potassium carbonate / N,N-dimethyl-formamide / 24 h / 0 - 20 °C / Inert atmosphere 2.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione; fluorobenzene / dimethyl sulfoxide / 24 h / 85 °C / Inert atmosphere 3.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 24 h / 0 - 20 °C / Inert atmosphere 3.2: 90 °C / Inert atmosphere; Darkness 3.3: 18 h / 37 °C / Inert atmosphere 4.1: potassium iodide / N,N-dimethyl-formamide / 16 h / 120 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 4 steps 1.1: potassium carbonate / N,N-dimethyl-formamide / 24 h / 0 - 20 °C / Inert atmosphere 2.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione; fluorobenzene / dimethyl sulfoxide / 24 h / 85 °C / Inert atmosphere 3.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 24 h / 0 - 20 °C / Inert atmosphere 3.2: 90 °C / Inert atmosphere; Darkness 4.1: potassium iodide / N,N-dimethyl-formamide / 16 h / 120 °C / Inert atmosphere View Scheme |
-
-
69660-90-8
(4aS,6aS,6bR,8aR,12aR,12bR,14bS)-methyl2,2,6a,6b,9,9,12a-heptamethyl-10-oxo-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,12a,12b,13,14b-octadecahydropicene-4a-carboxylate

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 24 h / 0 - 20 °C / Inert atmosphere 1.2: 24 h / 35 °C / Inert atmosphere 2.1: potassium iodide / N,N-dimethyl-formamide / 24 h / 120 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 2 steps 1.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 24 h / 0 - 20 °C / Inert atmosphere 1.2: 90 °C / Inert atmosphere; Darkness 1.3: 18 h / 37 °C / Inert atmosphere 2.1: potassium iodide / N,N-dimethyl-formamide / 16 h / 120 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1: 3-chloro-benzenecarboperoxoic acid / dichloromethane 2: hydrogen bromide; bromine; acetic acid 3: potassium iodide / N,N-dimethyl-formamide / 120 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 3-chloro-benzenecarboperoxoic acid / dichloromethane 2: hydrogen bromide; bromine; acetic acid 3: potassium iodide / N,N-dimethyl-formamide / 120 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: 3-chloro-benzenecarboperoxoic acid; hydrogen bromide / dichloromethane / 26 h / 20 °C 1.2: 24 h / 35 °C 2.1: potassium iodide / N,N-dimethyl-formamide / 30 h / 120 °C View Scheme |
-
-
1724-17-0, 73584-64-2
oleanolic acid methyl ester

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione; fluorobenzene / dimethyl sulfoxide / 24 h / 85 °C / Inert atmosphere 2.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 24 h / 0 - 20 °C / Inert atmosphere 2.2: 24 h / 35 °C / Inert atmosphere 3.1: potassium iodide / N,N-dimethyl-formamide / 24 h / 120 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione; fluorobenzene / dimethyl sulfoxide / 24 h / 85 °C / Inert atmosphere 2.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 24 h / 0 - 20 °C / Inert atmosphere 2.2: 90 °C / Inert atmosphere; Darkness 2.3: 18 h / 37 °C / Inert atmosphere 3.1: potassium iodide / N,N-dimethyl-formamide / 16 h / 120 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione; fluorobenzene / dimethyl sulfoxide / 24 h / 85 °C / Inert atmosphere 2.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 24 h / 0 - 20 °C / Inert atmosphere 2.2: 90 °C / Inert atmosphere; Darkness 3.1: potassium iodide / N,N-dimethyl-formamide / 16 h / 120 °C / Inert atmosphere View Scheme |
-
-
65023-20-3
methyl 3β-acetoxy-12-oxoolean-9(11)-en-28-oate

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 97 percent / methanolic KOH / 0.5 h / Heating 2: 92 percent / Jones reagent / acetone / 0.17 h / 20 °C 3: 99 percent / NaOMe / benzene / 2 h / 20 °C 4: 66 percent / NH2OH*HCl / ethanol; H2O / 1 h / Heating 5: 100 percent / NaOMe / methanol; diethyl ether / 0.75 h / 20 °C 6: 92 percent / DDQ / benzene / 0.5 h / Heating View Scheme | |
| Multi-step reaction with 6 steps 1: aq. KOH / methanol 2: CrO3, H2SO4 3: 100 percent / sodium methoxide / benzene 4: 61 percent / NH2OH*HCl / aq. ethanol 5: 100 percent / sodium methoxide / methanol; diethyl ether 6: 1.) phenylselenenyl chloride, 2.) 30 percent aq. H2O2 / 1.) ethyl acetate, 2.) THF View Scheme | |
| Multi-step reaction with 5 steps 1: potassium hydroxide; water / methanol 2: sodium methylate / benzene 3: hydroxylamine hydrochloride / water; ethanol 4: sodium methylate / methanol; diethyl ether 5: Phenylselenyl chloride / ethyl acetate View Scheme | |
| Multi-step reaction with 4 steps 1.1: potassium hydroxide / methanol / 1 h / Reflux 2.1: chromium(VI) oxide; sulfuric acid / water; acetone / 0 - 20 °C 3.1: lithium diisopropyl amide / tetrahydrofuran; n-heptane; ethylbenzene / 0.33 h / -78 - 20 °C / Inert atmosphere 3.2: 0.08 h / -78 - 20 °C 4.1: 2,3-dicyano-5,6-dichloro-p-benzoquinone / benzene / 0.5 h / Reflux; Inert atmosphere View Scheme |
-
-
218600-50-1
methyl 3,12-dioxoolean-9(11)-en-28-oate

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 99 percent / NaOMe / benzene / 2 h / 20 °C 2: 66 percent / NH2OH*HCl / ethanol; H2O / 1 h / Heating 3: 100 percent / NaOMe / methanol; diethyl ether / 0.75 h / 20 °C 4: 92 percent / DDQ / benzene / 0.5 h / Heating View Scheme | |
| Multi-step reaction with 4 steps 1: 100 percent / sodium methoxide / benzene 2: 61 percent / NH2OH*HCl / aq. ethanol 3: 100 percent / sodium methoxide / methanol; diethyl ether 4: 1.) phenylselenenyl chloride, 2.) 30 percent aq. H2O2 / 1.) ethyl acetate, 2.) THF View Scheme | |
| Multi-step reaction with 4 steps 1: sodium methylate / benzene 2: hydroxylamine hydrochloride / water; ethanol 3: sodium methylate / methanol; diethyl ether 4: Phenylselenyl chloride / ethyl acetate View Scheme | |
| Multi-step reaction with 2 steps 1.1: lithium diisopropyl amide / tetrahydrofuran; n-heptane; ethylbenzene / 0.33 h / -78 - 20 °C / Inert atmosphere 1.2: 0.08 h / -78 - 20 °C 2.1: 2,3-dicyano-5,6-dichloro-p-benzoquinone / benzene / 0.5 h / Reflux; Inert atmosphere View Scheme |
-
-
65023-19-0
methyl 3β-hydroxy-12-oxoolean-9(11)-en-28-oate

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 92 percent / Jones reagent / acetone / 0.17 h / 20 °C 2: 99 percent / NaOMe / benzene / 2 h / 20 °C 3: 66 percent / NH2OH*HCl / ethanol; H2O / 1 h / Heating 4: 100 percent / NaOMe / methanol; diethyl ether / 0.75 h / 20 °C 5: 92 percent / DDQ / benzene / 0.5 h / Heating View Scheme | |
| Multi-step reaction with 5 steps 1: CrO3, H2SO4 2: 100 percent / sodium methoxide / benzene 3: 61 percent / NH2OH*HCl / aq. ethanol 4: 100 percent / sodium methoxide / methanol; diethyl ether 5: 1.) phenylselenenyl chloride, 2.) 30 percent aq. H2O2 / 1.) ethyl acetate, 2.) THF View Scheme | |
| Multi-step reaction with 3 steps 1.1: chromium(VI) oxide; sulfuric acid / water; acetone / 0 - 20 °C 2.1: lithium diisopropyl amide / tetrahydrofuran; n-heptane; ethylbenzene / 0.33 h / -78 - 20 °C / Inert atmosphere 2.2: 0.08 h / -78 - 20 °C 3.1: 2,3-dicyano-5,6-dichloro-p-benzoquinone / benzene / 0.5 h / Reflux; Inert atmosphere View Scheme |
-
-
218600-52-3
methyl 12-oxoisoxazolo[4,5-b]olean-9(11)-en-28-oate

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 100 percent / NaOMe / methanol; diethyl ether / 0.75 h / 20 °C 2: 92 percent / DDQ / benzene / 0.5 h / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: 100 percent / sodium methoxide / methanol; diethyl ether 2: 1.) phenylselenenyl chloride, 2.) 30 percent aq. H2O2 / 1.) ethyl acetate, 2.) THF View Scheme |
-
-
305818-39-7
methyl 2-hydroxymethylene-3,12-dioxoolean-9(11)-en-28-oate

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 66 percent / NH2OH*HCl / ethanol; H2O / 1 h / Heating 2: 100 percent / NaOMe / methanol; diethyl ether / 0.75 h / 20 °C 3: 92 percent / DDQ / benzene / 0.5 h / Heating View Scheme |
-
-
218600-51-2
(4aS,6aR,6bS,8aR,12aS,14aR,14bS)-11-Formyl-2,2,6a,6b,9,9,12a-heptamethyl-10,14-dioxo-1,3,4,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-octadecahydro-2H-picene-4a-carboxylic acid methyl ester

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 61 percent / NH2OH*HCl / aq. ethanol 2: 100 percent / sodium methoxide / methanol; diethyl ether 3: 1.) phenylselenenyl chloride, 2.) 30 percent aq. H2O2 / 1.) ethyl acetate, 2.) THF View Scheme | |
| Multi-step reaction with 3 steps 1: hydroxylamine hydrochloride / water; ethanol 2: sodium methylate / methanol; diethyl ether 3: Phenylselenyl chloride / ethyl acetate View Scheme |
-
-
25493-69-0, 122798-61-2, 1721-57-9
(4aS,6aS,6bR,10S,12aR)-methyl 10-acetoxy-2,2,6a,6b,9,9,12a-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylate

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 0 - 20 °C 2.1: hydrogen bromide; bromine / acetonitrile / 19 h / 35 °C 3.1: potassium hydroxide / methanol / 1 h / Reflux 4.1: chromium(VI) oxide; sulfuric acid / water; acetone / 0 - 20 °C 5.1: lithium diisopropyl amide / tetrahydrofuran; n-heptane; ethylbenzene / 0.33 h / -78 - 20 °C / Inert atmosphere 5.2: 0.08 h / -78 - 20 °C 6.1: 2,3-dicyano-5,6-dichloro-p-benzoquinone / benzene / 0.5 h / Reflux; Inert atmosphere View Scheme | |
| Multi-step reaction with 6 steps 1.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 12 h / 0 - 20 °C 2.1: hydrogen bromide; bromine / acetonitrile / 19 h / 35 °C 3.1: potassium hydroxide / methanol / 1 h / Reflux 4.1: chromium(VI) oxide; sulfuric acid / water; acetone / 0 - 20 °C 5.1: lithium diisopropyl amide / tetrahydrofuran; n-heptane; ethylbenzene / 0.33 h / -78 - 20 °C / Inert atmosphere 5.2: 0.08 h / -78 - 20 °C 6.1: 2,3-dicyano-5,6-dichloro-p-benzoquinone / benzene / 0.5 h / Reflux; Inert atmosphere View Scheme |
-
-
4339-72-4
oleanolic acid 3-acetate

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: potassium carbonate / acetone / 24 h / 20 °C 2.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 0 - 20 °C 3.1: hydrogen bromide; bromine / acetonitrile / 19 h / 35 °C 4.1: potassium hydroxide / methanol / 1 h / Reflux 5.1: chromium(VI) oxide; sulfuric acid / water; acetone / 0 - 20 °C 6.1: lithium diisopropyl amide / tetrahydrofuran; n-heptane; ethylbenzene / 0.33 h / -78 - 20 °C / Inert atmosphere 6.2: 0.08 h / -78 - 20 °C 7.1: 2,3-dicyano-5,6-dichloro-p-benzoquinone / benzene / 0.5 h / Reflux; Inert atmosphere View Scheme | |
| Multi-step reaction with 7 steps 1.1: potassium carbonate / acetone / 24 h / 20 °C 2.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 12 h / 0 - 20 °C 3.1: hydrogen bromide; bromine / acetonitrile / 19 h / 35 °C 4.1: potassium hydroxide / methanol / 1 h / Reflux 5.1: chromium(VI) oxide; sulfuric acid / water; acetone / 0 - 20 °C 6.1: lithium diisopropyl amide / tetrahydrofuran; n-heptane; ethylbenzene / 0.33 h / -78 - 20 °C / Inert atmosphere 6.2: 0.08 h / -78 - 20 °C 7.1: 2,3-dicyano-5,6-dichloro-p-benzoquinone / benzene / 0.5 h / Reflux; Inert atmosphere View Scheme |
-
-
25493-94-1
methyl 3-O-β-acetyl-11-dehydro-12-oxo-18β-oleanolate

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: hydrogen bromide; bromine / acetonitrile / 19 h / 35 °C 2.1: potassium hydroxide / methanol / 1 h / Reflux 3.1: chromium(VI) oxide; sulfuric acid / water; acetone / 0 - 20 °C 4.1: lithium diisopropyl amide / tetrahydrofuran; n-heptane; ethylbenzene / 0.33 h / -78 - 20 °C / Inert atmosphere 4.2: 0.08 h / -78 - 20 °C 5.1: 2,3-dicyano-5,6-dichloro-p-benzoquinone / benzene / 0.5 h / Reflux; Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| Stage #1: bardoxolone With oxalyl dichloride; N,N-dimethyl-formamide In dichloromethane at 20℃; for 1.16667h; Cooling with ice; Stage #2: methanol In dichloromethane for 1h; | 96% |
-
-
305818-40-0
1,2-dihydro-2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid methyl ester

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In benzene for 0.5h; Heating; | 92% |

| Conditions | Yield |
|---|---|
| With potassium iodide In N,N-dimethyl-formamide at 120℃; for 16h; Inert atmosphere; | 89% |

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| With porcine liver esterase; γ-glutamyl transpeptidase In aq. buffer at 37℃; for 8h; pH=7.4; Enzymatic reaction; | 88% |

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In benzene for 0.5h; Reflux; Inert atmosphere; | 87% |
| Stage #1: methyl 2-cyano-3,12-dioxoolean-9(11)-en-28-oate With Phenylselenyl chloride In ethyl acetate Stage #2: With dihydrogen peroxide In tetrahydrofuran; water | 40% |
| With Phenylselenyl chloride; dihydrogen peroxide 1.) ethyl acetate, 2.) THF; Yield given; Multistep reaction; |
-
-
1428550-98-4
2-bromo-3,12-dioxo-oleanane-1,9 (11)-diene-28-carboxylic acid methyl ester

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| With potassium iodide In N,N-dimethyl-formamide at 120℃; for 30h; | 83.6% |
| With potassium iodide In N,N-dimethyl-formamide at 120℃; | 73% |
| With potassium iodide In N,N-dimethyl-formamide at 120℃; | 73% |
| With potassium iodide In N,N-dimethyl-formamide at 120℃; for 24h; Temperature; Time; Reagent/catalyst; Inert atmosphere; | 66% |
-
-
65023-19-0
methyl 3β-hydroxy-12-oxoolean-9(11)-en-28-oate

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 92 percent / Jones reagent / acetone / 0.17 h / 20 °C 2: 99 percent / NaOMe / benzene / 2 h / 20 °C 3: 66 percent / NH2OH*HCl / ethanol; H2O / 1 h / Heating 4: 100 percent / NaOMe / methanol; diethyl ether / 0.75 h / 20 °C 5: 92 percent / DDQ / benzene / 0.5 h / Heating View Scheme | |
| Multi-step reaction with 5 steps 1: CrO3, H2SO4 2: 100 percent / sodium methoxide / benzene 3: 61 percent / NH2OH*HCl / aq. ethanol 4: 100 percent / sodium methoxide / methanol; diethyl ether 5: 1.) phenylselenenyl chloride, 2.) 30 percent aq. H2O2 / 1.) ethyl acetate, 2.) THF View Scheme | |
| Multi-step reaction with 3 steps 1.1: chromium(VI) oxide; sulfuric acid / water; acetone / 0 - 20 °C 2.1: lithium diisopropyl amide / tetrahydrofuran; n-heptane; ethylbenzene / 0.33 h / -78 - 20 °C / Inert atmosphere 2.2: 0.08 h / -78 - 20 °C 3.1: 2,3-dicyano-5,6-dichloro-p-benzoquinone / benzene / 0.5 h / Reflux; Inert atmosphere View Scheme |
-
-
65023-20-3
methyl 3β-acetoxy-12-oxoolean-9(11)-en-28-oate

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 97 percent / methanolic KOH / 0.5 h / Heating 2: 92 percent / Jones reagent / acetone / 0.17 h / 20 °C 3: 99 percent / NaOMe / benzene / 2 h / 20 °C 4: 66 percent / NH2OH*HCl / ethanol; H2O / 1 h / Heating 5: 100 percent / NaOMe / methanol; diethyl ether / 0.75 h / 20 °C 6: 92 percent / DDQ / benzene / 0.5 h / Heating View Scheme | |
| Multi-step reaction with 6 steps 1: aq. KOH / methanol 2: CrO3, H2SO4 3: 100 percent / sodium methoxide / benzene 4: 61 percent / NH2OH*HCl / aq. ethanol 5: 100 percent / sodium methoxide / methanol; diethyl ether 6: 1.) phenylselenenyl chloride, 2.) 30 percent aq. H2O2 / 1.) ethyl acetate, 2.) THF View Scheme | |
| Multi-step reaction with 5 steps 1: potassium hydroxide; water / methanol 2: sodium methylate / benzene 3: hydroxylamine hydrochloride / water; ethanol 4: sodium methylate / methanol; diethyl ether 5: Phenylselenyl chloride / ethyl acetate View Scheme | |
| Multi-step reaction with 4 steps 1.1: potassium hydroxide / methanol / 1 h / Reflux 2.1: chromium(VI) oxide; sulfuric acid / water; acetone / 0 - 20 °C 3.1: lithium diisopropyl amide / tetrahydrofuran; n-heptane; ethylbenzene / 0.33 h / -78 - 20 °C / Inert atmosphere 3.2: 0.08 h / -78 - 20 °C 4.1: 2,3-dicyano-5,6-dichloro-p-benzoquinone / benzene / 0.5 h / Reflux; Inert atmosphere View Scheme |
-
-
218600-50-1
methyl 3,12-dioxoolean-9(11)-en-28-oate

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 99 percent / NaOMe / benzene / 2 h / 20 °C 2: 66 percent / NH2OH*HCl / ethanol; H2O / 1 h / Heating 3: 100 percent / NaOMe / methanol; diethyl ether / 0.75 h / 20 °C 4: 92 percent / DDQ / benzene / 0.5 h / Heating View Scheme | |
| Multi-step reaction with 4 steps 1: 100 percent / sodium methoxide / benzene 2: 61 percent / NH2OH*HCl / aq. ethanol 3: 100 percent / sodium methoxide / methanol; diethyl ether 4: 1.) phenylselenenyl chloride, 2.) 30 percent aq. H2O2 / 1.) ethyl acetate, 2.) THF View Scheme | |
| Multi-step reaction with 4 steps 1: sodium methylate / benzene 2: hydroxylamine hydrochloride / water; ethanol 3: sodium methylate / methanol; diethyl ether 4: Phenylselenyl chloride / ethyl acetate View Scheme | |
| Multi-step reaction with 2 steps 1.1: lithium diisopropyl amide / tetrahydrofuran; n-heptane; ethylbenzene / 0.33 h / -78 - 20 °C / Inert atmosphere 1.2: 0.08 h / -78 - 20 °C 2.1: 2,3-dicyano-5,6-dichloro-p-benzoquinone / benzene / 0.5 h / Reflux; Inert atmosphere View Scheme |
-
-
218600-52-3
methyl 12-oxoisoxazolo[4,5-b]olean-9(11)-en-28-oate

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 100 percent / NaOMe / methanol; diethyl ether / 0.75 h / 20 °C 2: 92 percent / DDQ / benzene / 0.5 h / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: 100 percent / sodium methoxide / methanol; diethyl ether 2: 1.) phenylselenenyl chloride, 2.) 30 percent aq. H2O2 / 1.) ethyl acetate, 2.) THF View Scheme |
-
-
305818-39-7
methyl 2-hydroxymethylene-3,12-dioxoolean-9(11)-en-28-oate

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 66 percent / NH2OH*HCl / ethanol; H2O / 1 h / Heating 2: 100 percent / NaOMe / methanol; diethyl ether / 0.75 h / 20 °C 3: 92 percent / DDQ / benzene / 0.5 h / Heating View Scheme |
-
-
218600-51-2
(4aS,6aR,6bS,8aR,12aS,14aR,14bS)-11-Formyl-2,2,6a,6b,9,9,12a-heptamethyl-10,14-dioxo-1,3,4,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-octadecahydro-2H-picene-4a-carboxylic acid methyl ester

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 61 percent / NH2OH*HCl / aq. ethanol 2: 100 percent / sodium methoxide / methanol; diethyl ether 3: 1.) phenylselenenyl chloride, 2.) 30 percent aq. H2O2 / 1.) ethyl acetate, 2.) THF View Scheme | |
| Multi-step reaction with 3 steps 1: hydroxylamine hydrochloride / water; ethanol 2: sodium methylate / methanol; diethyl ether 3: Phenylselenyl chloride / ethyl acetate View Scheme |
-
-
69660-90-8
(4aS,6aS,6bR,8aR,12aR,12bR,14bS)-methyl2,2,6a,6b,9,9,12a-heptamethyl-10-oxo-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,12a,12b,13,14b-octadecahydropicene-4a-carboxylate

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 24 h / 0 - 20 °C / Inert atmosphere 1.2: 24 h / 35 °C / Inert atmosphere 2.1: potassium iodide / N,N-dimethyl-formamide / 24 h / 120 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 2 steps 1.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 24 h / 0 - 20 °C / Inert atmosphere 1.2: 90 °C / Inert atmosphere; Darkness 1.3: 18 h / 37 °C / Inert atmosphere 2.1: potassium iodide / N,N-dimethyl-formamide / 16 h / 120 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1: 3-chloro-benzenecarboperoxoic acid / dichloromethane 2: hydrogen bromide; bromine; acetic acid 3: potassium iodide / N,N-dimethyl-formamide / 120 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 3-chloro-benzenecarboperoxoic acid / dichloromethane 2: hydrogen bromide; bromine; acetic acid 3: potassium iodide / N,N-dimethyl-formamide / 120 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: 3-chloro-benzenecarboperoxoic acid; hydrogen bromide / dichloromethane / 26 h / 20 °C 1.2: 24 h / 35 °C 2.1: potassium iodide / N,N-dimethyl-formamide / 30 h / 120 °C View Scheme |
-
-
508-02-1
Oleanolic acid

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: potassium carbonate / N,N-dimethyl-formamide / 24 h / 0 - 20 °C / Inert atmosphere 2.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione; fluorobenzene / dimethyl sulfoxide / 24 h / 85 °C / Inert atmosphere 3.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 24 h / 0 - 20 °C / Inert atmosphere 3.2: 24 h / 35 °C / Inert atmosphere 4.1: potassium iodide / N,N-dimethyl-formamide / 24 h / 120 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 4 steps 1.1: potassium carbonate / N,N-dimethyl-formamide / 24 h / 0 - 20 °C / Inert atmosphere 2.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione; fluorobenzene / dimethyl sulfoxide / 24 h / 85 °C / Inert atmosphere 3.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 24 h / 0 - 20 °C / Inert atmosphere 3.2: 90 °C / Inert atmosphere; Darkness 3.3: 18 h / 37 °C / Inert atmosphere 4.1: potassium iodide / N,N-dimethyl-formamide / 16 h / 120 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 4 steps 1.1: potassium carbonate / N,N-dimethyl-formamide / 24 h / 0 - 20 °C / Inert atmosphere 2.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione; fluorobenzene / dimethyl sulfoxide / 24 h / 85 °C / Inert atmosphere 3.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 24 h / 0 - 20 °C / Inert atmosphere 3.2: 90 °C / Inert atmosphere; Darkness 4.1: potassium iodide / N,N-dimethyl-formamide / 16 h / 120 °C / Inert atmosphere View Scheme |
-
-
1724-17-0, 73584-64-2
oleanolic acid methyl ester

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione; fluorobenzene / dimethyl sulfoxide / 24 h / 85 °C / Inert atmosphere 2.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 24 h / 0 - 20 °C / Inert atmosphere 2.2: 24 h / 35 °C / Inert atmosphere 3.1: potassium iodide / N,N-dimethyl-formamide / 24 h / 120 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione; fluorobenzene / dimethyl sulfoxide / 24 h / 85 °C / Inert atmosphere 2.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 24 h / 0 - 20 °C / Inert atmosphere 2.2: 90 °C / Inert atmosphere; Darkness 2.3: 18 h / 37 °C / Inert atmosphere 3.1: potassium iodide / N,N-dimethyl-formamide / 16 h / 120 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione; fluorobenzene / dimethyl sulfoxide / 24 h / 85 °C / Inert atmosphere 2.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 24 h / 0 - 20 °C / Inert atmosphere 2.2: 90 °C / Inert atmosphere; Darkness 3.1: potassium iodide / N,N-dimethyl-formamide / 16 h / 120 °C / Inert atmosphere View Scheme |
-
-
25493-69-0, 122798-61-2, 1721-57-9
(4aS,6aS,6bR,10S,12aR)-methyl 10-acetoxy-2,2,6a,6b,9,9,12a-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylate

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 0 - 20 °C 2.1: hydrogen bromide; bromine / acetonitrile / 19 h / 35 °C 3.1: potassium hydroxide / methanol / 1 h / Reflux 4.1: chromium(VI) oxide; sulfuric acid / water; acetone / 0 - 20 °C 5.1: lithium diisopropyl amide / tetrahydrofuran; n-heptane; ethylbenzene / 0.33 h / -78 - 20 °C / Inert atmosphere 5.2: 0.08 h / -78 - 20 °C 6.1: 2,3-dicyano-5,6-dichloro-p-benzoquinone / benzene / 0.5 h / Reflux; Inert atmosphere View Scheme | |
| Multi-step reaction with 6 steps 1.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 12 h / 0 - 20 °C 2.1: hydrogen bromide; bromine / acetonitrile / 19 h / 35 °C 3.1: potassium hydroxide / methanol / 1 h / Reflux 4.1: chromium(VI) oxide; sulfuric acid / water; acetone / 0 - 20 °C 5.1: lithium diisopropyl amide / tetrahydrofuran; n-heptane; ethylbenzene / 0.33 h / -78 - 20 °C / Inert atmosphere 5.2: 0.08 h / -78 - 20 °C 6.1: 2,3-dicyano-5,6-dichloro-p-benzoquinone / benzene / 0.5 h / Reflux; Inert atmosphere View Scheme |
-
-
25493-94-1
methyl 3-O-β-acetyl-11-dehydro-12-oxo-18β-oleanolate

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: hydrogen bromide; bromine / acetonitrile / 19 h / 35 °C 2.1: potassium hydroxide / methanol / 1 h / Reflux 3.1: chromium(VI) oxide; sulfuric acid / water; acetone / 0 - 20 °C 4.1: lithium diisopropyl amide / tetrahydrofuran; n-heptane; ethylbenzene / 0.33 h / -78 - 20 °C / Inert atmosphere 4.2: 0.08 h / -78 - 20 °C 5.1: 2,3-dicyano-5,6-dichloro-p-benzoquinone / benzene / 0.5 h / Reflux; Inert atmosphere View Scheme |
-
-
4339-72-4
oleanolic acid 3-acetate

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: potassium carbonate / acetone / 24 h / 20 °C 2.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 0 - 20 °C 3.1: hydrogen bromide; bromine / acetonitrile / 19 h / 35 °C 4.1: potassium hydroxide / methanol / 1 h / Reflux 5.1: chromium(VI) oxide; sulfuric acid / water; acetone / 0 - 20 °C 6.1: lithium diisopropyl amide / tetrahydrofuran; n-heptane; ethylbenzene / 0.33 h / -78 - 20 °C / Inert atmosphere 6.2: 0.08 h / -78 - 20 °C 7.1: 2,3-dicyano-5,6-dichloro-p-benzoquinone / benzene / 0.5 h / Reflux; Inert atmosphere View Scheme | |
| Multi-step reaction with 7 steps 1.1: potassium carbonate / acetone / 24 h / 20 °C 2.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 12 h / 0 - 20 °C 3.1: hydrogen bromide; bromine / acetonitrile / 19 h / 35 °C 4.1: potassium hydroxide / methanol / 1 h / Reflux 5.1: chromium(VI) oxide; sulfuric acid / water; acetone / 0 - 20 °C 6.1: lithium diisopropyl amide / tetrahydrofuran; n-heptane; ethylbenzene / 0.33 h / -78 - 20 °C / Inert atmosphere 6.2: 0.08 h / -78 - 20 °C 7.1: 2,3-dicyano-5,6-dichloro-p-benzoquinone / benzene / 0.5 h / Reflux; Inert atmosphere View Scheme |
-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 20℃; for 3h; Cooling with ice; | 99% |
| With dihydrogen peroxide In water; acetonitrile at 20℃; for 48h; | 92% |
| With iodosylbenzene In chloroform for 12h; Inert atmosphere; | 75% |
-
-
100-39-0
benzyl bromide

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| Stage #1: bardoxolone methyl With potassium carbonate In water; N,N-dimethyl-formamide at 20℃; Inert atmosphere; Stage #2: benzyl bromide In water; N,N-dimethyl-formamide at 20℃; for 2h; Inert atmosphere; | 84% |
-
-
218600-53-4
bardoxolone methyl

-
-
218600-44-3
bardoxolone

| Conditions | Yield |
|---|---|
| With lithium iodide In N,N-dimethyl-formamide | 71% |
| With lithium iodide In N,N-dimethyl-formamide | 71% |
| With lithium iodide In N,N-dimethyl-formamide at 153℃; for 12h; Inert atmosphere; | 70.3% |
| With lithium iodide In N,N-dimethyl-formamide for 4h; Heating; | 68% |
| With lithium iodide In N,N-dimethyl-formamide Inert atmosphere; Reflux; | 44% |
-
-
218600-53-4
bardoxolone methyl

-
-
1198076-72-0
(4aS,6aR,6bS,8aR,11R,12R,12aS,14aR,14bS)-methyl-11-cyano-11,12-dihydroxy-2,2,6a,6b,9,9,12a-heptamethyl-10,14-dioxo-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-icosahydropicene-4a-carboxylate

| Conditions | Yield |
|---|---|
| With sodium periodate; (x)ClH*Cl3Ru In water; ethyl acetate; acetonitrile at 0℃; for 0.0833333h; | 71% |
-
-
67-56-1
methanol

-
-
138500-85-3
4-bromomethylphenylboronic acid pinacol ester

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| Stage #1: methanol; bardoxolone methyl With potassium carbonate In N,N-dimethyl-formamide at 20℃; Stage #2: 4-bromomethylphenylboronic acid pinacol ester In N,N-dimethyl-formamide for 0.5h; | 67% |

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| With iodosylbenzene In chloroform at 20℃; for 16h; | 62% |

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| Stage #1: bardoxolone methyl With potassium carbonate In water; N,N-dimethyl-formamide at 20℃; Inert atmosphere; Stage #2: methyl N2-(((9H-fluoren-9-yl)methoxy)carbonyl)-N5-(4-(bromomethyl)phenyl)-L-glutaminate In water; N,N-dimethyl-formamide at 20℃; for 0.5h; Inert atmosphere; | 61% |
| Stage #1: bardoxolone methyl With water; potassium carbonate In N,N-dimethyl-formamide at 20℃; Stage #2: methyl N2-(((9H-fluoren-9-yl)methoxy)carbonyl)-N5-(4-(bromomethyl)phenyl)-L-glutaminate In N,N-dimethyl-formamide for 0.5h; | 53% |

| Conditions | Yield |
|---|---|
| Stage #1: bardoxolone methyl With water; potassium carbonate In N,N-dimethyl-formamide at 20℃; Stage #2: 4-bromomethylphenylboronic acid pinacol ester In N,N-dimethyl-formamide for 0.5h; | 53% |
-
-
64-17-5
ethanol

-
-
138500-85-3
4-bromomethylphenylboronic acid pinacol ester

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| Stage #1: ethanol; bardoxolone methyl With potassium carbonate In N,N-dimethyl-formamide at 20℃; Stage #2: 4-bromomethylphenylboronic acid pinacol ester In N,N-dimethyl-formamide for 0.5h; | 49% |

-
-
64-17-5
ethanol

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| Stage #1: ethanol; bardoxolone methyl With potassium carbonate In N,N-dimethyl-formamide at 20℃; Stage #2: methyl N2-(((9H-fluoren-9-yl)methoxy)carbonyl)-N5-(4-(bromomethyl)phenyl)-L-glutaminate In N,N-dimethyl-formamide for 0.5h; | 39% |

-
-
67-56-1
methanol

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| Stage #1: methanol; bardoxolone methyl With potassium carbonate In N,N-dimethyl-formamide at 20℃; Stage #2: methyl N2-(((9H-fluoren-9-yl)methoxy)carbonyl)-N5-(4-(bromomethyl)phenyl)-L-glutaminate In N,N-dimethyl-formamide for 0.5h; | 38% |


| Conditions | Yield |
|---|---|
| Stage #1: isosorbide mononitrate; bardoxolone methyl With potassium carbonate In N,N-dimethyl-formamide at 20℃; Stage #2: acetyl chloride In N,N-dimethyl-formamide for 6h; | 35% |


| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide In tetrahydrofuran at -78℃; Inert atmosphere; | 16% |
-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| Stage #1: bardoxolone methyl With N-bromosaccharin; water In acetonitrile at 20℃; for 2h; Stage #2: With triethylamine In benzene at 20℃; for 1h; | 4.9% |
-
-
218600-53-4
bardoxolone methyl

-
-
71-43-2
benzene

| Conditions | Yield |
|---|---|
| In acetone for 0.666667h; Ultrasonic processor; |
-
-
67-56-1
methanol

-
-
218600-53-4
bardoxolone methyl

| Conditions | Yield |
|---|---|
| at -10 - 60℃; | |
| at -15 - 60℃; Product distribution / selectivity; Industry scale; |
Related products
Raw Materials
Downstream Products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View