Simagchem Corporation
Welcome to Simagchem, your partner in China as a premier supply of bulk specialty chemicals for industry and life science. We introduce experienced quality product and exceptional JIT service with instant market intelligence in China to benefit our
Cas:274693-55-9
Min.Order:0 Metric Ton
Negotiable
Type:Manufacturers
inquiryAlity Chemical Corporation
The above product is Ality Chemical's strong item with best price, good quality and fast supply. Ality Chemical has been focusing on the research and production of this field for over 14 years. At the same time, we are always committed to providi
Cas:274693-55-9
Min.Order:1
Negotiable
Type:Other
inquiryEnke Pharma-tech Co.,Ltd. (Cangzhou, China )
Cangzhou Enke Pharma Tech Co.,ltd. is located in Cangzhou City, Hebei province ,where is a famous petroleum chemical industry city in China. Enke Pharma a high-tech enterprise ,and we are dedicated to developing and manufacturing new api, intermedi
Cas:274693-55-9
Min.Order:1 Kilogram
FOB Price: $1.0
Type:Manufacturers
inquiryDayang Chem (Hangzhou) Co.,Ltd.
As a leading manufacturer and supplier of chemicals in China, DayangChem not only supply popular chemicals, but also DayangChem's R&D center offer custom synthesis services. DayangChem can provide different quantities of custom synthesis ch
Cas:274693-55-9
Min.Order:1 Kilogram
FOB Price: $3.0
Type:Lab/Research institutions
inquiryChemwill Asia Co., Ltd.
Our main production base is located in Xuzhou industry park. We are certified both to the ISO 9001 and ISO 14001 Standards, have a safety management system in place.Our R&D team masters core technology for process-design of target building block
Cas:274693-55-9
Min.Order:5 Kiloliter
FOB Price: $1.2 / 5.0
Type:Manufacturers
inquiryHenan Tianfu Chemical Co., Ltd.
Our company was built in 2009 with an ISO certificate.In the past 5 years, we have grown up as a famous fine chemicals supplier in China and we had established stable business relationships with Samsung,LG,Merck,Thermo Fisher Scientific and so on.O
Cas:274693-55-9
Min.Order:1 Kilogram
FOB Price: $1.0
Type:Lab/Research institutions
inquiryZhuozhou Wenxi import and Export Co., Ltd
WITH US,YOUR MONEY IN SAFE,YOUR BUSINESS IN SAFE 1)Quick Response Within 12 hours; 2)Quality Guarantee: All products are strictly tested by our QC, confirmed by QA and approved by third party lab in China, USA, Canada, Germany, UK, Italy, France et
Cas:274693-55-9
Min.Order:1 Kilogram
FOB Price: $139.0 / 210.0
Type:Trading Company
inquiryShanghai Upbio Tech Co.,Ltd
1.No Less 8 years exporting experience. Clients can 100% received goods 2.Lower Price with higher quality 3,Free sample 4,We are sincerely responsible for the "product quality" and "After Service" Upbio is Specialized
Cas:274693-55-9
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryBaoji Guokang Healthchem co.,ltd
Our company has been in existence for 10 years since its establishment. We have our own unique team. The company integrates independent research and development, production and sales. We have established famous brands at home and abroad. At prese
Cas:274693-55-9
Min.Order:1 Kilogram
FOB Price: $51.0 / 65.0
Type:Trading Company
inquiryQingdao Beluga Import and Export Co., LTD
1-Acetyladamantane Intermediate CAS:274693-55-9 Qingdao Belugas Import and Export Co., Ltd. is a scientific and technological company integrating research and development, production and trade of chemical intermediates, specializing in high quality
Cas:274693-55-9
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryShandong Hanjiang Chemical Co., Ltd.
Hello, dear friend! I'm Hansen and Allen from China. Welcome to my lookchem mall! The following is a brief introduction of our company's products and services. If you are interested in our products, please contact us by emai
Cas:274693-55-9
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryHebei Quanhe Biotechnology Co. LTD
1. Timely and efficient service to ensure communication with customers 2. Produce products of different specifications and sizes according to your requirements. 3. Quality procedures and standards recognized by SGS. Advanced plant equipment ensures s
Cas:274693-55-9
Min.Order:1 Kilogram
FOB Price: $30.0 / 50.0
Type:Lab/Research institutions
inquiryHenan Wentao Chemical Product Co., Ltd.
Henan Wentao Chemical Product Co.,Ltd is Located in Zhengzhou High-tech Development Zone with import and export license, We passed ISO 9001:2008 as well, Henan Wentao has developed more than 1000 compounds, which are widely used in the fields of prod
Cas:274693-55-9
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryXiamen Hisunny Chemical Co.,Ltd
Best quality & Attractive price & Professional service; Trial & Pilot & Commercial Hisunny Chemical is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality intermediates, specia
Cas:274693-55-9
Min.Order:0
Negotiable
Type:Manufacturers
inquiryWuhan Han Sheng New Material Technology Co.,Ltd
Our Advantage: high quality with competitive price High quality standard: BP/USP/EP Enterprise standard All purity customized Fast and safe delivery We have reliable forwarder who can help us deliver our goods more fast and safe. We
Cas:274693-55-9
Min.Order:10 Kilogram
Negotiable
Type:Trading Company
inquiryTriumph International Development Limilted
Triumph has the complete production of G- KG - MT service chain,we can make the new technology into productivity quickly in the research and development of new products. Main Service 1.Own made fine chemical products 2.Out sourcing and qua
Cas:274693-55-9
Min.Order:100 Gram
Negotiable
Type:Lab/Research institutions
inquiryHebei Nengqian Chemical Import and Export Co., LTD
Our Advantage Rich Experience Our products are sold all over Europe,North&South America, Sino-East, Asia and pacific area as well as Africa,we establish long term. Quality service Company cooperates with research institutes. We strictly con
Cas:274693-55-9
Min.Order:1 Kilogram
FOB Price: $15.0 / 50.0
Type:Trading Company
inquiryHANWAYS CHEMPHARM CO.,LIMITED
We are concentrating on APIs and pharmaceutical intermediates. With in depth knowledge and understanding in this industry, we are indulged in supplying a wide assortments of pharmaceutical intermediates from Wuhan, Hubei, China. It is made ob
Cas:274693-55-9
Min.Order:1 Kilogram
Negotiable
Type:Trading Company
inquiryAfine Chemicals Limited
Company Introduction 1. Established in 2005, with two independent business divisions: Fine chemicals division; Pharmaceutical division. 2. Main product: Optical brightener Textile auxiliary Dye stuff Pigments
Cas:274693-55-9
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryHangzhou Keyingchem Co.,Ltd
Hangzhou KeyingChem Co., Ltd. exported this product to many countries and regions at best price. If you are looking for the material’s manufacturer or supplier in China, KeyingChem is your best choice. Pls contact with us freely for getting det
Cas:274693-55-9
Min.Order:0 Metric Ton
Negotiable
Type:Lab/Research institutions
inquirySHANGHAI T&W PHARMACEUTICAL CO., LTD.
A substitute for perfluorooctanoic acid, mainly used as a surfactant, dispersant, additive, etc Appearance:White solid or Colorless liquid Purity:99.3 % We will ship the goods in a timely manner as required We can provide relevant documents acc
Cas:274693-55-9
Min.Order:4 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryShanghai Hope Chem Co., Ltd
1. Product advantages: exquisite appearance and unique functions. 2. Product Advantages: Our products are the best,fast speed ,in large stock 3. High-quality products and thoughtful service are your greatest satisfaction. 4. The product
Cas:274693-55-9
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryHangzhou J&H Chemical Co., Ltd.
J&H CHEM R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. J&H CHEM has some Manufacturing base in Jia
Cas:274693-55-9
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryShanghai Massive Chemical Technology Co., Ltd.
Massive Chemical is certified with ISO9001 and ISO14001 manufacturer for this product. We will offer all documents as requirement for the materials which includes, Certificate of Analysis, Material Safety Data Sheet, and Method of Analysis and
Cas:274693-55-9
Min.Order:1 Gram
FOB Price: $1.0
Type:Lab/Research institutions
inquiryZibo Hangyu Biotechnology Development Co., Ltd
Zibo Hangyu Biotechnology Development Co., Ltd is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemi
Cas:274693-55-9
Min.Order:10 Gram
FOB Price: $100.0
Type:Lab/Research institutions
inquiryHANGZHOU YUNUO CHEMICAL CO.,LTD
Superior quality, moderate price & quick delivery. Appearance:white powder Storage:Stored in cool, dry and ventilation place; Away from fire and heat Package:as per your request Application:Used as Pharmaceutical Intermediates Transportation:as
Cas:274693-55-9
Min.Order:1 Gram
Negotiable
Type:Trading Company
inquirySinoway Industrial Co., Ltd.
Why is SINOWAY: 1) Specialized in pharmaceutical and healthcare industrial for 34 years. 2) ISO 9001:2015 & SGS audited supplier . 3) Accept various payment terms : T.T 30-60 days. 4) We have warehouse in USA with quickly shipment . 5) We c
Cas:274693-55-9
Min.Order:1 Kilogram
Negotiable
Type:Trading Company
inquirySiwei Development Group Ltd.
Product name: 2-((3aR,4S,6R,6aS)-6-Amino-2,2-dimethyltetrahydro-3aH-Cyclopenta[d][1,3]dioxol-4-yloxy)ethanol L-Tataric Acid CAS No.:274693-55-9 Molecule Formula:C10H19NO4 Molecule Weight:217.26 Purity: 99.0% Package: 25kg/drum Descripti
Cas:274693-55-9
Min.Order:1 Kilogram
Negotiable
Type:Trading Company
inquiryZibo Dorne chemical technology co. LTD
Product Details Grade: pharmaceutical grade Purity:99%+ ProductionCapacity: 1000 Kilogram/Month Scope of use: For scientific research only(The product must be used legally) Our Advantage 1. Best quality with competitive price. 2. Quick shipping,
Cas:274693-55-9
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryShanghai Minstar Chemical Co., Ltd
1-Acetyladamantane Intermediate Basic information Product Name: 1-Acetyladamantane Intermediate Synonyms: 2-(6-Amino-2,2-dimethyl-tetrahydro-cyclopenta[1,3]dioxol-4-yloxy)-ethanol;2-((3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopent
Cas:274693-55-9
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquirySynthetic route
-
-
274693-54-8
benzyl 6-(2-hydroxyethoxy)-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-ylcarbamate

-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In ethanol | 99% |
| With 5%-palladium/activated carbon In methanol at 20℃; for 1h; | 75.7% |
| palladium on charcoal In ethanol |

-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen In ethanol at 50 - 60℃; under 750.075 Torr; for 12h; Autoclave; Inert atmosphere; | 98% |
| With palladium on activated charcoal; hydrogen; acetic acid In methanol at 20℃; under 3750.38 - 6000.6 Torr; for 10h; | 92.8% |

-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| With hydrogenchloride; pyridoxal 5'-phosphate; isopropylamine; triethylamine In water at 25℃; for 16h; pH=8; Solvent; Reagent/catalyst; Temperature; Large scale; Enzymatic reaction; | 97% |

-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| In ethylene glycol at 120℃; for 8h; Solvent; Temperature; | 92% |

-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen; acetic acid In methanol at 20℃; for 14h; | 91.5% |
-
-
1444301-50-1
(3aS,4R,6S,6aR)-N-benzyl-6-(2-(benzyloxy)ethoxy)-2,2-dimethyl-tetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-amine

-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In methanol at 40℃; under 7500.75 Torr; for 168h; | 63% |
-
-
345898-95-5
N-(((4S,5R)-2,2-dimethyl-5-vinyl-1 ,3-dioxolan-4-yl)methylene)-1-phenylmethanamine oxide

-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: N,N-dimethyl-formamide; toluene / 5 h / 110 °C 2.1: palladium 10% on activated carbon; hydrogen / methanol / 14 h / 20 °C 3.1: N-ethyl-N,N-diisopropylamine / 4-methyl-2-pentanone / 2 h / 20 °C 4.1: sodium hydride / N,N-dimethyl-formamide / 0.5 h / -30 °C 4.2: 3 h / 20 °C 5.1: lithium borohydride / tetrahydrofuran / 5 h / 0 °C 6.1: 5%-palladium/activated carbon / methanol / 1 h / 20 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: toluene / 5 h / Reflux 2.1: zinc; acetic acid / diethyl ether / 72.48 h / 0 - 25 °C 3.1: sodium hydride / N,N-dimethyl-formamide / 0.5 h / 0 °C / Inert atmosphere 3.2: 4 h / 0 - 20 °C / Inert atmosphere 4.1: palladium 10% on activated carbon; hydrogen / methanol / 168 h / 40 °C / 7500.75 Torr View Scheme | |
| Multi-step reaction with 6 steps 1.1: N,N-dimethyl-formamide / 2 h / Reflux 2.1: ammonium formate; palladium 10% on activated carbon / methanol / 1.5 h / Reflux 3.1: potassium carbonate / water / 5.5 h / 20 °C 4.1: sodium hydride / N,N-dimethyl-formamide / 1 h / -20 °C 4.2: 6.33 h / -20 - 20 °C 5.1: sodium tetrahydroborate; ethanol / 4 h / 60 °C 6.1: ammonium formate; palladium 10% on activated carbon / ethanol / 1 h / 40 °C View Scheme |

-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: lithium borohydride / tetrahydrofuran / 5 h / 0 °C 2: 5%-palladium/activated carbon / methanol / 1 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: lithium borohydride / tetrahydrofuran / 0 - 20 °C 1.2: 20 °C 1.3: pH 6 - 7 2.1: ammonium formate / palladium 10% on activated carbon / ethanol / 30 - 70 °C View Scheme |
-
-
274693-53-7
[3aS-(3aα,4α,6α,6aα)]-(tetrahydro-6-hydroxy-2,2-dimethyl-4H-cyclopenta-1,3-dioxol-4-yl)carbamic acid phenylmethyl ester

-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: sodium hydride / N,N-dimethyl-formamide / 0.5 h / -30 °C 1.2: 3 h / 20 °C 2.1: lithium borohydride / tetrahydrofuran / 5 h / 0 °C 3.1: 5%-palladium/activated carbon / methanol / 1 h / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: potassium tert-butylate / tetrahydrofuran / 0 °C 2.1: lithium borohydride / tetrahydrofuran / 0 - 20 °C 2.2: 20 °C 2.3: pH 6 - 7 3.1: ammonium formate / palladium 10% on activated carbon / ethanol / 30 - 70 °C View Scheme | |
| Multi-step reaction with 2 steps 1: potassium tert-butylate 2: hydrogen / palladium on activated charcoal / ethanol View Scheme | |
| Multi-step reaction with 3 steps 1.1: sodium hydride / N,N-dimethyl-formamide / 1 h / -20 °C 1.2: 6.33 h / -20 - 20 °C 2.1: sodium tetrahydroborate; ethanol / 4 h / 60 °C 3.1: ammonium formate; palladium 10% on activated carbon / ethanol / 1 h / 40 °C View Scheme |
-
-
4099-85-8, 5531-21-5, 20672-63-3, 20672-65-5, 58763-00-1, 63029-07-2, 72402-14-3, 84894-45-1, 129263-68-9
methyl 2,3-O-isopropylidene-D-ribofuranoside

-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1.1: pyridine / 24 h / 20 °C 2.1: sodium iodide / butanone / 24 h / 120 °C 3.1: zinc / ethanol / 1 h / 70 °C 4.1: sodium carbonate / ethanol / 1 h / 50 °C 5.1: N,N-dimethyl-formamide; toluene / 5 h / 110 °C 6.1: palladium 10% on activated carbon; hydrogen / methanol / 14 h / 20 °C 7.1: N-ethyl-N,N-diisopropylamine / 4-methyl-2-pentanone / 2 h / 20 °C 8.1: sodium hydride / N,N-dimethyl-formamide / 0.5 h / -30 °C 8.2: 3 h / 20 °C 9.1: lithium borohydride / tetrahydrofuran / 5 h / 0 °C 10.1: 5%-palladium/activated carbon / methanol / 1 h / 20 °C View Scheme | |
| Multi-step reaction with 8 steps 1.1: pyridine / 4 h / -10 - -5 °C 2.1: lithium bromide / butanone / 22 h / 80 °C 3.1: hydrogenchloride; zinc/copper couple / water; methanol / 18 h / 25 °C 4.1: sodium carbonate / methanol / 0.5 h / 25 °C 5.1: toluene / 5 h / Reflux 6.1: zinc; acetic acid / diethyl ether / 72.48 h / 0 - 25 °C 7.1: sodium hydride / N,N-dimethyl-formamide / 0.5 h / 0 °C / Inert atmosphere 7.2: 4 h / 0 - 20 °C / Inert atmosphere 8.1: palladium 10% on activated carbon; hydrogen / methanol / 168 h / 40 °C / 7500.75 Torr View Scheme |
-
-
532-20-7, 613-83-2, 7261-25-8, 7687-39-0, 13221-22-2, 14795-83-6, 15761-67-8, 20074-49-1, 25545-03-3, 25545-04-4, 32445-75-3, 34436-17-4, 36441-93-7, 36468-53-8, 37110-85-3, 37388-49-1, 38029-69-5, 40461-77-6, 40461-89-0, 41546-19-4, 41546-20-7, 41546-21-8, 41546-26-3, 41546-29-6, 41546-30-9, 41546-31-0, 126872-16-0, 131064-98-7
D-Ribose

-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1.1: perchloric acid / acetone / 24 h / Molecular sieve 2.1: pyridine / 24 h / 20 °C 3.1: sodium iodide / butanone / 24 h / 120 °C 4.1: zinc / ethanol / 1 h / 70 °C 5.1: sodium carbonate / ethanol / 1 h / 50 °C 6.1: N,N-dimethyl-formamide; toluene / 5 h / 110 °C 7.1: palladium 10% on activated carbon; hydrogen / methanol / 14 h / 20 °C 8.1: N-ethyl-N,N-diisopropylamine / 4-methyl-2-pentanone / 2 h / 20 °C 9.1: sodium hydride / N,N-dimethyl-formamide / 0.5 h / -30 °C 9.2: 3 h / 20 °C 10.1: lithium borohydride / tetrahydrofuran / 5 h / 0 °C 11.1: 5%-palladium/activated carbon / methanol / 1 h / 20 °C View Scheme |
-
-
4137-56-8, 5531-22-6, 6953-71-5, 52631-00-2, 63087-95-6, 84894-43-9, 13007-50-6
((3aR,4R,6aR)-6-methoxy-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methyl 4-methylbenzenesulfonate

-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1.1: sodium iodide / butanone / 24 h / 120 °C 2.1: zinc / ethanol / 1 h / 70 °C 3.1: sodium carbonate / ethanol / 1 h / 50 °C 4.1: N,N-dimethyl-formamide; toluene / 5 h / 110 °C 5.1: palladium 10% on activated carbon; hydrogen / methanol / 14 h / 20 °C 6.1: N-ethyl-N,N-diisopropylamine / 4-methyl-2-pentanone / 2 h / 20 °C 7.1: sodium hydride / N,N-dimethyl-formamide / 0.5 h / -30 °C 7.2: 3 h / 20 °C 8.1: lithium borohydride / tetrahydrofuran / 5 h / 0 °C 9.1: 5%-palladium/activated carbon / methanol / 1 h / 20 °C View Scheme | |
| Multi-step reaction with 7 steps 1.1: lithium bromide / butanone / 22 h / 80 °C 2.1: hydrogenchloride; zinc/copper couple / water; methanol / 18 h / 25 °C 3.1: sodium carbonate / methanol / 0.5 h / 25 °C 4.1: toluene / 5 h / Reflux 5.1: zinc; acetic acid / diethyl ether / 72.48 h / 0 - 25 °C 6.1: sodium hydride / N,N-dimethyl-formamide / 0.5 h / 0 °C / Inert atmosphere 6.2: 4 h / 0 - 20 °C / Inert atmosphere 7.1: palladium 10% on activated carbon; hydrogen / methanol / 168 h / 40 °C / 7500.75 Torr View Scheme |
-
-
155934-55-7
(4R,5R)-2,2-dimethyl-5-vinyl-[1,3]dioxolane-4-carbaldehyde

-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: sodium carbonate / ethanol / 1 h / 50 °C 2.1: N,N-dimethyl-formamide; toluene / 5 h / 110 °C 3.1: palladium 10% on activated carbon; hydrogen / methanol / 14 h / 20 °C 4.1: N-ethyl-N,N-diisopropylamine / 4-methyl-2-pentanone / 2 h / 20 °C 5.1: sodium hydride / N,N-dimethyl-formamide / 0.5 h / -30 °C 5.2: 3 h / 20 °C 6.1: lithium borohydride / tetrahydrofuran / 5 h / 0 °C 7.1: 5%-palladium/activated carbon / methanol / 1 h / 20 °C View Scheme | |
| Multi-step reaction with 5 steps 1.1: sodium carbonate / methanol / 0.5 h / 25 °C 2.1: toluene / 5 h / Reflux 3.1: zinc; acetic acid / diethyl ether / 72.48 h / 0 - 25 °C 4.1: sodium hydride / N,N-dimethyl-formamide / 0.5 h / 0 °C / Inert atmosphere 4.2: 4 h / 0 - 20 °C / Inert atmosphere 5.1: palladium 10% on activated carbon; hydrogen / methanol / 168 h / 40 °C / 7500.75 Torr View Scheme |
-
-
155899-66-4
(3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-ol

-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: N-ethyl-N,N-diisopropylamine / 4-methyl-2-pentanone / 2 h / 20 °C 2.1: sodium hydride / N,N-dimethyl-formamide / 0.5 h / -30 °C 2.2: 3 h / 20 °C 3.1: lithium borohydride / tetrahydrofuran / 5 h / 0 °C 4.1: 5%-palladium/activated carbon / methanol / 1 h / 20 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: potassium carbonate / ethanol; water / 3 h / 60 - 65 °C 1.2: 0.25 h 2.1: sodium t-butanolate / N,N-dimethyl-formamide; tetrahydrofuran / 2 h / -10 - 5 °C 3.1: diisobutylaluminium hydride / toluene / 2.5 h / -25 - -20 °C 3.2: 0.25 h 4.1: hydrogen / 20% palladium hydroxide-activated charcoal / methanol / 10 h / 20 - 25 °C / 2327.23 Torr / Autoclave; Inert atmosphere View Scheme | |
| Multi-step reaction with 4 steps 1.1: sodium carbonate / water; 4-methyl-2-pentanone / 20 °C 2.1: potassium tert-butylate / tetrahydrofuran / 0 °C 3.1: lithium borohydride / tetrahydrofuran / 0 - 20 °C 3.2: 20 °C 3.3: pH 6 - 7 4.1: ammonium formate / palladium 10% on activated carbon / ethanol / 30 - 70 °C View Scheme |
-
-
155855-51-9
tetrahydro-2,2-dimethyl-6-phenylmethyl-(3aS,4S,7R,7aS)-4,7-methano-4H-1,3-dioxolo[4,5-d][1,2]oxazine

-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: palladium 10% on activated carbon; hydrogen / methanol / 14 h / 20 °C 2.1: N-ethyl-N,N-diisopropylamine / 4-methyl-2-pentanone / 2 h / 20 °C 3.1: sodium hydride / N,N-dimethyl-formamide / 0.5 h / -30 °C 3.2: 3 h / 20 °C 4.1: lithium borohydride / tetrahydrofuran / 5 h / 0 °C 5.1: 5%-palladium/activated carbon / methanol / 1 h / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: zinc; acetic acid / diethyl ether / 72.48 h / 0 - 25 °C 2.1: sodium hydride / N,N-dimethyl-formamide / 0.5 h / 0 °C / Inert atmosphere 2.2: 4 h / 0 - 20 °C / Inert atmosphere 3.1: palladium 10% on activated carbon; hydrogen / methanol / 168 h / 40 °C / 7500.75 Torr View Scheme |
-
-
50600-40-3
(3aS,4S,6aR)-4-(iodomethyl)-6-methoxy-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxole

-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1.1: zinc / ethanol / 1 h / 70 °C 2.1: sodium carbonate / ethanol / 1 h / 50 °C 3.1: N,N-dimethyl-formamide; toluene / 5 h / 110 °C 4.1: palladium 10% on activated carbon; hydrogen / methanol / 14 h / 20 °C 5.1: N-ethyl-N,N-diisopropylamine / 4-methyl-2-pentanone / 2 h / 20 °C 6.1: sodium hydride / N,N-dimethyl-formamide / 0.5 h / -30 °C 6.2: 3 h / 20 °C 7.1: lithium borohydride / tetrahydrofuran / 5 h / 0 °C 8.1: 5%-palladium/activated carbon / methanol / 1 h / 20 °C View Scheme |
-
-
1383715-55-6
(3aR,4S,6R,6aS)-6-(N,N-dibenzylamino)-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-ol

-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: sodium t-butanolate / N,N-dimethyl-formamide; tetrahydrofuran / 2 h / -10 - 5 °C 2.1: diisobutylaluminium hydride / toluene / 2.5 h / -25 - -20 °C 2.2: 0.25 h 3.1: hydrogen / 20% palladium hydroxide-activated charcoal / methanol / 10 h / 20 - 25 °C / 2327.23 Torr / Autoclave; Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1.1: potassium tert-butylate / dichloromethane; tetrahydrofuran / 5.5 h / 0 - 5 °C 2.1: hydrogen / palladium-on-charcoal / ethanol / 14 h / 43 - 48 °C / 3620.13 Torr / Autoclave 3.1: lithium borohydride / dichloromethane / 24 h / 20 - 25 °C / Inert atmosphere 3.2: 0.25 h 3.3: 0.5 h View Scheme | |
| Multi-step reaction with 4 steps 1.1: potassium tert-butylate / dichloromethane; tetrahydrofuran / 5.5 h / 0 - 5 °C 2.1: palladium 10% on activated carbon; hydrogen / ethanol-d6 / 14 h / 43 - 48 °C / 3620.13 Torr / Inert atmosphere 2.2: -5 - 55 °C 3.1: potassium carbonate / water; dichloromethane / 20 - 25 °C / pH 10 - 10.5 4.1: lithium borohydride / dichloromethane / 24 h / 20 - 25 °C / pH 10 - 10.5 / Inert atmosphere View Scheme |
-
-
1383715-56-7
tert-butyl [[(3aR,4S,6R,6aS)-6-(N,N-dibenzylamino)-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yl]oxy]acetate

-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: diisobutylaluminium hydride / toluene / 2.5 h / -25 - -20 °C 1.2: 0.25 h 2.1: hydrogen / 20% palladium hydroxide-activated charcoal / methanol / 10 h / 20 - 25 °C / 2327.23 Torr / Autoclave; Inert atmosphere View Scheme | |
| Multi-step reaction with 2 steps 1.1: hydrogen / palladium-on-charcoal / ethanol / 14 h / 43 - 48 °C / 3620.13 Torr / Autoclave 2.1: lithium borohydride / dichloromethane / 24 h / 20 - 25 °C / Inert atmosphere 2.2: 0.25 h 2.3: 0.5 h View Scheme | |
| Multi-step reaction with 3 steps 1.1: palladium 10% on activated carbon; hydrogen / ethanol-d6 / 14 h / 43 - 48 °C / 3620.13 Torr / Inert atmosphere 1.2: -5 - 55 °C 2.1: potassium carbonate / water; dichloromethane / 20 - 25 °C / pH 10 - 10.5 3.1: lithium borohydride / dichloromethane / 24 h / 20 - 25 °C / pH 10 - 10.5 / Inert atmosphere View Scheme |
-
-
1383715-57-8
2-[[(3aR,4S,6R,6aS)-6-(N,N-dibenzylamino)-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yl]oxy]ethanol

-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| With hydrogen; 20% palladium hydroxide-activated charcoal In methanol at 20 - 25℃; under 2327.23 Torr; for 10h; Autoclave; Inert atmosphere; |
-
-
88756-83-6
(+/-)-1β-amino-2α,3α-O-isopropylidene-2α,3α,4β-cyclopentanetriol

-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: ethanol / 20 °C / Reflux; Resolution of racemate 2: potassium carbonate / water; 4-methyl-2-pentanone 3: potassium tert-butylate 4: hydrogen / palladium on activated charcoal / ethanol View Scheme | |
| Multi-step reaction with 4 steps 1: ethanol / 20 °C / Reflux; Resolution of racemate 2: potassium carbonate / water; 4-methyl-2-pentanone 3: potassium tert-butylate 4: hydrogen / palladium on activated charcoal / ethanol View Scheme |
-
-
1392909-30-6
(3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta-[d][1,3]dioxol-4-ol D-(-)-mandelate

-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: potassium carbonate / water; 4-methyl-2-pentanone 2: potassium tert-butylate 3: hydrogen / palladium on activated charcoal / ethanol View Scheme |
-
-
1392909-34-0
(3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta-[d][1,3]dioxol-4-ol R-(-)-3-chloromandelate

-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: potassium carbonate / water; 4-methyl-2-pentanone 2: potassium tert-butylate 3: hydrogen / palladium on activated charcoal / ethanol View Scheme |
-
-
1416158-39-8
tert-butyl [[(3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yl]oxy]acetate

-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| Stage #1: tert-butyl [[(3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yl]oxy]acetate With lithium borohydride In dichloromethane at 20 - 25℃; for 24h; Inert atmosphere; Stage #2: With acetic acid In dichloromethane for 0.25h; Stage #3: With potassium carbonate In dichloromethane for 0.5h; | |
| With lithium borohydride In dichloromethane at 20 - 25℃; for 24h; pH=10 - 10.5; Inert atmosphere; | 0.7 g |
-
-
78341-96-5
(3aS,4S,6aR)-4-(bromomethyl)-6-methoxy-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxole

-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: hydrogenchloride; zinc/copper couple / water; methanol / 18 h / 25 °C 2.1: sodium carbonate / methanol / 0.5 h / 25 °C 3.1: toluene / 5 h / Reflux 4.1: zinc; acetic acid / diethyl ether / 72.48 h / 0 - 25 °C 5.1: sodium hydride / N,N-dimethyl-formamide / 0.5 h / 0 °C / Inert atmosphere 5.2: 4 h / 0 - 20 °C / Inert atmosphere 6.1: palladium 10% on activated carbon; hydrogen / methanol / 168 h / 40 °C / 7500.75 Torr View Scheme |
-
-
155855-53-1
(3aR,4S,6R,6aS)-6-(benzylamino)-2,2-dimethyltetrahydro-3ah-cyclopenta-[d][1,3]-dioxol-4-ol

-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: sodium hydride / N,N-dimethyl-formamide / 0.5 h / 0 °C / Inert atmosphere 1.2: 4 h / 0 - 20 °C / Inert atmosphere 2.1: palladium 10% on activated carbon; hydrogen / methanol / 168 h / 40 °C / 7500.75 Torr View Scheme |
-
-
10257-32-6
D-ribose

-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1.1: hydrogenchloride / acetone / 22 h / 25 °C 2.1: pyridine / 4 h / -10 - -5 °C 3.1: lithium bromide / butanone / 22 h / 80 °C 4.1: hydrogenchloride; zinc/copper couple / water; methanol / 18 h / 25 °C 5.1: sodium carbonate / methanol / 0.5 h / 25 °C 6.1: toluene / 5 h / Reflux 7.1: zinc; acetic acid / diethyl ether / 72.48 h / 0 - 25 °C 8.1: sodium hydride / N,N-dimethyl-formamide / 0.5 h / 0 °C / Inert atmosphere 8.2: 4 h / 0 - 20 °C / Inert atmosphere 9.1: palladium 10% on activated carbon; hydrogen / methanol / 168 h / 40 °C / 7500.75 Torr View Scheme | |
| Multi-step reaction with 9 steps 1.1: Dowex WX8 / 72 h / 25 °C 2.1: pyridine / 4 h / -10 - -5 °C 3.1: lithium bromide / butanone / 22 h / 80 °C 4.1: hydrogenchloride; zinc/copper couple / water; methanol / 18 h / 25 °C 5.1: sodium carbonate / methanol / 0.5 h / 25 °C 6.1: toluene / 5 h / Reflux 7.1: zinc; acetic acid / diethyl ether / 72.48 h / 0 - 25 °C 8.1: sodium hydride / N,N-dimethyl-formamide / 0.5 h / 0 °C / Inert atmosphere 8.2: 4 h / 0 - 20 °C / Inert atmosphere 9.1: palladium 10% on activated carbon; hydrogen / methanol / 168 h / 40 °C / 7500.75 Torr View Scheme |

-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium hydrogencarbonate / water; ethyl acetate / 1 h / 20 °C 2: palladium 10% on activated carbon; hydrogen / methanol / 168 h / 40 °C / 7500.75 Torr View Scheme |
-
-
1416158-40-1
tert-butyl [[(3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yl]oxy]acetate L-(+)-tartaric acid salt

-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: potassium carbonate / water; dichloromethane / 20 - 25 °C / pH 10 - 10.5 2: lithium borohydride / dichloromethane / 24 h / 20 - 25 °C / pH 10 - 10.5 / Inert atmosphere View Scheme |

-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: ammonium formate; palladium 10% on activated carbon / methanol / 1.5 h / Reflux 2.1: potassium carbonate / water / 5.5 h / 20 °C 3.1: sodium hydride / N,N-dimethyl-formamide / 1 h / -20 °C 3.2: 6.33 h / -20 - 20 °C 4.1: sodium tetrahydroborate; ethanol / 4 h / 60 °C 5.1: ammonium formate; palladium 10% on activated carbon / ethanol / 1 h / 40 °C View Scheme | |
| Multi-step reaction with 3 steps 1: zinc; acetic acid / tetrahydrofuran / 24 h / 5 - 25 °C 2: Nafion-H / toluene / 5 h / Reflux 3: palladium on activated charcoal; hydrogen / ethanol / 12 h / 50 - 60 °C / 750.08 Torr / Autoclave; Inert atmosphere View Scheme |
-
-
145783-15-9
4,6-dichloro-2-(propylsulfanyl)-5-pyrimidinamine

-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| Stage #1: 4,6-dichloro-2-(propylsulfanyl)-5-pyrimidinamine; [3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol In water; N,N-dimethyl-formamide for 0.333333h; Stage #2: With triethylamine at 20℃; Reflux; | 95% |
| With N-ethyl-N,N-diisopropylamine at 120 - 125℃; for 10h; Temperature; Inert atmosphere; | 93.5% |
| With sodium hydrogencarbonate In water at 100℃; for 20h; Reagent/catalyst; Temperature; | 88.3% |
-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

-
-
145783-14-8
4,6-dichloro-5-nitro-2-(propylsulfanyl)pyrimidine

-
-
1265919-25-2
2-[((3aR,4S,6R,6aS)-6-{[5-nitro-6-chloro-2-(propylthio)-4-pyrimidinyl]amino}-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yl)oxy]-1-ethanol

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 10 - 20℃; for 2h; | 93.5% |
| In tetrahydrofuran at 0 - 10℃; for 2h; | 45% |
-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| With triflic azide; potassium carbonate; copper(II) sulfate In acetonitrile at 0 - 20℃; for 3h; Inert atmosphere; | 92.6% |
-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

-
-
384-98-5
2,4,6-trimethylbenzene-1-sulfonyl fluoride

| Conditions | Yield |
|---|---|
| With 1,1,3,3-Tetramethyldisiloxane; benzotriazol-1-ol; N-ethyl-N,N-diisopropylamine In dimethyl sulfoxide at 25℃; for 24h; Sealed tube; | 92% |
-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

-
-
91322-00-8
5-amino-2,4,6-trichloropyrimidine

| Conditions | Yield |
|---|---|
| With triethylamine at 110℃; Sealed tube; | 83% |
-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

-
-
1444301-60-3
(1S,2S,3R,5S)-3-amino-5-(2-hydroxyethoxy)cyclopentane-1,2-diol

| Conditions | Yield |
|---|---|
| Stage #1: [3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol With hydrogenchloride In methanol; water at 20℃; for 24h; Stage #2: With sodium carbonate In isopropyl alcohol at 20℃; for 24h; | 80% |
| With hydrogenchloride In methanol; water at 10℃; for 11h; | 4.0 g |
-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| With triethylamine In pentan-1-ol at 115℃; for 10h; | 78.75% |
-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In tetrahydrofuran at 35℃; for 8h; | 75.05% |
-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

-
-
4359-87-9
2,4,6-trichloro-5-nitropyrimidine

| Conditions | Yield |
|---|---|
| With sodium carbonate In tetrahydrofuran at 20℃; for 4h; | 59% |
-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

-
-
144-62-7
oxalic acid

-
-
1402150-30-4
2-[[(3aR,4S,6R,6aS)-6-amino-2,2-dimethyl-tetrahydro-3aH-cyclopenta[d][1,3]-dioxol-4-yl]oxy]-1-ethanol oxalate

| Conditions | Yield |
|---|---|
| In ethanol; water at 65℃; |
-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

-
-
2743-38-6
O,O'-dibenzoyl-L-tartaric acid

| Conditions | Yield |
|---|---|
| In ethanol at 10 - 50℃; for 4h; |
-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: triethylamine; sodium iodide / 1,4-dioxane / 7 h / 120 °C 2: sodium nitrite / water; acetic acid; ethyl acetate / 30 °C View Scheme | |
| Multi-step reaction with 2 steps 1: potassium tert-butylate 2: isopentyl nitrite / acetonitrile View Scheme | |
| Multi-step reaction with 2 steps 1: sodium hydrogencarbonate / water / 20 h / 100 °C 2: sodium nitrite / water; acetic acid / 2 h / 0 °C View Scheme |
-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

-
-
274693-27-5
ticagrelor

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: triethylamine; sodium iodide / 1,4-dioxane / 7 h / 120 °C 2: sodium nitrite / water; acetic acid; ethyl acetate / 30 °C 3: N-ethyl-N,N-diisopropylamine / dichloromethane / 16 h 4: hydrogenchloride; water / methanol / 1 h View Scheme | |
| Multi-step reaction with 4 steps 1.1: N-ethyl-N,N-diisopropylamine / tetrahydrofuran / 2 h / 20 - 25 °C 2.1: sodium hydrogencarbonate; sodium dithionite / water; acetone / 2 h / 20 - 25 °C 3.1: sodium azide; acetic acid / toluene; water / 1 h / 5 - 10 °C 4.1: hydrogenchloride; water / toluene; methanol / 2 h / 25 - 30 °C 4.2: pH > 8 View Scheme | |
| Multi-step reaction with 5 steps 1.1: N-ethyl-N,N-diisopropylamine / tetrahydrofuran / 2 h / 20 - 25 °C 2.1: sodium hydrogencarbonate; sodium dithionite / water; acetone / 2 h / 20 - 25 °C 3.1: sodium azide; acetic acid / toluene; water / 1 h / 5 - 10 °C 4.1: potassium carbonate / acetone / 24 h / 25 - 60 °C 5.1: hydrogenchloride; water / methanol / 5.5 h / 20 - 55 °C 5.2: 25 - 30 °C / pH 10 View Scheme |
-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: triethylamine; sodium iodide / 1,4-dioxane / 7 h / 120 °C 2.1: sodium nitrite / water; acetic acid; ethyl acetate / 30 °C 3.1: N-ethyl-N,N-diisopropylamine / dichloromethane / 16 h 4.1: triethylamine / acetonitrile / 0.5 h / 20 °C 4.2: 2 h / 20 °C View Scheme |
-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: triethylamine; sodium iodide / 1,4-dioxane / 7 h / 120 °C 2.1: sodium nitrite / water; acetic acid; ethyl acetate / 30 °C 3.1: N-ethyl-N,N-diisopropylamine / dichloromethane / 16 h 4.1: triethylamine / acetonitrile / 0.5 h / 20 °C 4.2: 2 h / 20 °C View Scheme |
-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: triethylamine; sodium iodide / 1,4-dioxane / 7 h / 120 °C 2.1: sodium nitrite / water; acetic acid; ethyl acetate / 30 °C 3.1: N-ethyl-N,N-diisopropylamine / dichloromethane / 16 h 4.1: triethylamine / acetonitrile / 0.5 h / 20 °C 4.2: 2 h / 20 °C View Scheme |
-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

-
-
1381841-44-6
2-((1S,2S,3S,4R)-4-(7-(((1R,2S)-2-(3,4-difluorophenyl)cyclopropyl)amino-5-propylthio-3H-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl)-2,3-dihydroxycyclopentyl)oxy) thyl carbonate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: triethylamine; sodium iodide / 1,4-dioxane / 7 h / 120 °C 2.1: sodium nitrite / water; acetic acid; ethyl acetate / 30 °C 3.1: N-ethyl-N,N-diisopropylamine / dichloromethane / 16 h 4.1: triethylamine / acetonitrile / 0.5 h / 20 °C 4.2: 2 h / 20 °C 5.1: iodine / methanol / 4 h / 60 °C View Scheme |
-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

-
-
1381841-46-8
2-((1S,2S,3S,4R)-4-(7-(((1R,2S)-2-(3,4-difluorophenyl)cyclopropyl)amino-5-propylthio-3H-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl)-2,3-dihydroxycyclopentyl)oxy)ethyl isobutyl carbonate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: triethylamine; sodium iodide / 1,4-dioxane / 7 h / 120 °C 2.1: sodium nitrite / water; acetic acid; ethyl acetate / 30 °C 3.1: N-ethyl-N,N-diisopropylamine / dichloromethane / 16 h 4.1: triethylamine / acetonitrile / 0.5 h / 20 °C 4.2: 2 h / 20 °C 5.1: iodine / methanol / 4 h / 60 °C View Scheme |
-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

-
-
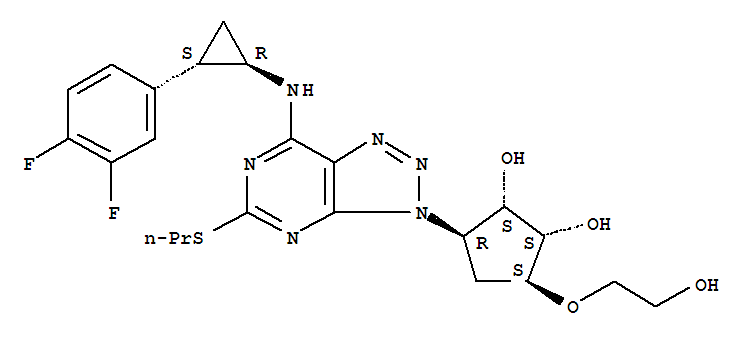
274693-26-4
[3aR-[3aα,4α,6α,(1R,2S),6aα]]-2-[6-[[[7-(2-(3,4-difluorophenyl)cyclopropyl)]amino-5-(propylthio)-3H-1,2,3-triazolo[4,5-d]pyrimidin-3-yl]tetrahydro-2,2-dimethyl-4H-cyclopenteno-1,3-dioxolan-4-yl]oxy]ethanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: triethylamine; sodium iodide / 1,4-dioxane / 7 h / 120 °C 2: sodium nitrite / water; acetic acid; ethyl acetate / 30 °C 3: N-ethyl-N,N-diisopropylamine / dichloromethane / 16 h View Scheme | |
| Multi-step reaction with 4 steps 1: N-ethyl-N,N-diisopropylamine / tetrahydrofuran / 2 h / 20 - 25 °C 2: sodium hydrogencarbonate; sodium dithionite / water; acetone / 2 h / 20 - 25 °C 3: sodium azide; acetic acid / toluene; water / 1 h / 5 - 10 °C 4: iodine / acetone / 2 h / 25 - 60 °C View Scheme | |
| Multi-step reaction with 3 steps 1: potassium tert-butylate 2: isopentyl nitrite / acetonitrile 3: N-ethyl-N,N-diisopropylamine View Scheme |
-
-
274693-55-9
[3aR-(3aα,4α,6α,6aα)]-2-[[6-amino-2,2-dimethyl tetrahydro-4H-cyclopenta-1,3-dioxol-4-yl]oxy]ethanol

-
-
1381841-39-9
2-((1S,2S,3S,4R)-4-(7-(((1R,2S)-2-(3,4-difluorophenyl)cyclopropyl)amino-5-propylthio-3H-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl)-2,3-dihydroxycyclopentyl)oxy)ethyl butyrate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: triethylamine; sodium iodide / 1,4-dioxane / 7 h / 120 °C 2.1: sodium nitrite / water; acetic acid; ethyl acetate / 30 °C 3.1: N-ethyl-N,N-diisopropylamine / dichloromethane / 16 h 4.1: triethylamine / acetonitrile / 0.5 h / 20 °C 4.2: 2 h / 20 °C 5.1: iodine / methanol / 4 h / 60 °C View Scheme |
Related products
Raw Materials
Downstream Products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View