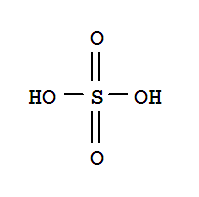
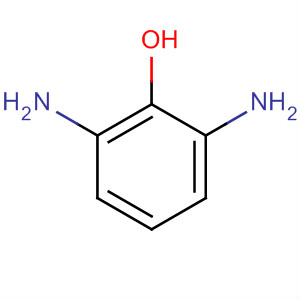
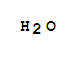This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
Synthetic route

| Conditions | Yield |
|---|---|
| With ammonium cerium(IV) nitrate In acetonitrile at 20℃; for 12h; | A 95% B 3% |

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride In dichloromethane at -5 - 25℃; | 92% |
-
-
18710-65-1
N-(2,6-dinitrophenyl)-α-alanine

-
B
-
573-56-8
2,6-dinitrophenol

| Conditions | Yield |
|---|---|
| With sodium hydroxide In 1,4-dioxane; water for 0.25h; Heating; | A 90% B n/a |

-
B
-
573-56-8
2,6-dinitrophenol

| Conditions | Yield |
|---|---|
| With sodium hydroxide In 1,4-dioxane; water for 0.333333h; Heating; | A 83% B 7% |

-
B
-
573-56-8
2,6-dinitrophenol

| Conditions | Yield |
|---|---|
| With sodium hydroxide In 1,4-dioxane; water for 0.166667h; Heating; | A 79% B 10% |

| Conditions | Yield |
|---|---|
| With perchloric acid; montmorillonite K10 supported ammonium nitrate at 50℃; for 1.5h; | A 73% B 23% |
| durch Nitrieren; Trennung durch fraktionierte Faellung des Gemisches der Kaliumsalze mit BaCl2; | |
| With tetrachloromethane; nitrosylsulfuric acid at 30℃; | |
| With nitric acid |

| Conditions | Yield |
|---|---|
| With thionyl chloride; bismuth subnitrate In dichloromethane at 20℃; for 2h; | A 72% B 14% |
-
-
71-43-2
benzene

-
A
-
51-28-5
2,4-Dinitrophenol

-
B
-
100-02-7
4-nitro-phenol

-
C
-
573-56-8
2,6-dinitrophenol

-
D
-
98-95-3
nitrobenzene

-
E
-
88-75-5
2-hydroxynitrobenzene

| Conditions | Yield |
|---|---|
| With nitric acid; 2,3-dicyano-5,6-dichloro-p-benzoquinone at 30 - 35℃; for 24h; Irradiation; | A n/a B n/a C 62% D n/a E n/a |


| Conditions | Yield |
|---|---|
| With uronium nitrate In water; acetonitrile at 80℃; for 1h; Microwave irradiation; regioselective reaction; | 60% |
| With sulfuric acid; nitric acid; acetic acid | |
| durch Nitrierung; |

| Conditions | Yield |
|---|---|
| With oxygen; nitric acid; 2,3-dicyano-5,6-dichloro-p-benzoquinone In water at 30℃; for 24h; Irradiation; Large scale; | 56% |
-
-
108-95-2
phenol

-
A
-
51-28-5
2,4-Dinitrophenol

-
B
-
100-02-7
4-nitro-phenol

-
C
-
573-56-8
2,6-dinitrophenol

-
D
-
88-75-5
2-hydroxynitrobenzene

| Conditions | Yield |
|---|---|
| With trinitratooxovanadium(V) In dichloromethane for 0.00833333h; Ambient temperature; Further byproducts given; | A 51% B 9% C 1% D 32% |

-
-
626-23-3
Di-sec-butylamin

-
-
573-55-7
2-fluoro-1,3-dinitrobenzene

-
-
584-08-7
potassium carbonate

-
A
-
573-56-8
2,6-dinitrophenol

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide for 3h; Heating; | A 45% B 48% |

-
-
573-55-7
2-fluoro-1,3-dinitrobenzene

-
-
584-08-7
potassium carbonate

-
-
108-18-9
diisopropylamine

-
A
-
573-56-8
2,6-dinitrophenol

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide for 3h; Heating; | A 29% B 42% |

-
-
69-72-7
salicylic acid

-
A
-
51-28-5
2,4-Dinitrophenol

-
B
-
573-56-8
2,6-dinitrophenol

-
C
-
96-97-9
5-nitrosalicylic acid

-
D
-
88-75-5
2-hydroxynitrobenzene

| Conditions | Yield |
|---|---|
| With ammonium cerium(IV) nitrate In acetonitrile at 50 - 60℃; for 0.25h; Further byproducts given; | A 24% B 13% C 37% D 5% |

-
-
69-72-7
salicylic acid

-
A
-
51-28-5
2,4-Dinitrophenol

-
B
-
573-56-8
2,6-dinitrophenol

-
C
-
85-38-1
3-nitrosalicylic acid

-
D
-
88-75-5
2-hydroxynitrobenzene

| Conditions | Yield |
|---|---|
| With ammonium cerium(IV) nitrate In acetonitrile at 50 - 60℃; for 0.25h; Further byproducts given; | A 24% B 13% C 31% D 5% |
| With ammonium cerium(IV) nitrate In acetonitrile at 50 - 60℃; for 0.25h; Further byproducts given; | A 24% B 13% C 24% D 5% |

-
-
50-78-2
aspirin

-
A
-
51-28-5
2,4-Dinitrophenol

-
B
-
573-56-8
2,6-dinitrophenol

-
C
-
85-38-1
3-nitrosalicylic acid

-
D
-
88-75-5
2-hydroxynitrobenzene

| Conditions | Yield |
|---|---|
| With ammonium cerium(IV) nitrate In acetonitrile at 60 - 70℃; for 0.25h; Further byproducts given; | A 24% B 13% C 31% D 5% |

-
-
50-78-2
aspirin

-
A
-
51-28-5
2,4-Dinitrophenol

-
B
-
573-56-8
2,6-dinitrophenol

-
C
-
96-97-9
5-nitrosalicylic acid

-
D
-
88-75-5
2-hydroxynitrobenzene

| Conditions | Yield |
|---|---|
| With ammonium cerium(IV) nitrate In acetonitrile at 60 - 70℃; for 0.25h; Further byproducts given; | A 24% B 13% C 31% D 5% |

-
-
509-14-8
tetranitromethane

-
-
71-43-2
benzene

-
A
-
573-56-8
2,6-dinitrophenol

-
B
-
71156-15-5
1-nitro-4-trinitromethylbenzene

-
C
-
67483-29-8
phenyltrinitromethane

-
D
-
88-89-1
2,4,6-Trinitrophenol

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 48h; Irradiation; Further byproducts given. Title compound not separated from byproducts; | A 0.6% B 3% C 3.3% D 0.3% |

-
-
509-14-8
tetranitromethane

-
-
71-43-2
benzene

-
A
-
573-56-8
2,6-dinitrophenol

-
B
-
67483-29-8
phenyltrinitromethane

-
C
-
88-89-1
2,4,6-Trinitrophenol

-
D
-
157096-63-4
1,3-dinitro-5-trinitromethylbenzene

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 48h; Irradiation; Further byproducts given. Title compound not separated from byproducts; | A 0.6% B 3.3% C 0.3% D 0.4% |


| Conditions | Yield |
|---|---|
| With sulfuric acid at 140 - 150℃; |
-
-
85365-92-0
3,5-dinitro-4-methoxybenzoic acid

-
-
573-56-8
2,6-dinitrophenol

| Conditions | Yield |
|---|---|
| With water at 170℃; |
-
-
17973-92-1
isopicramic acid

-
-
573-56-8
2,6-dinitrophenol

| Conditions | Yield |
|---|---|
| Kochen der Diazoniumverbindung mit absol. Alkohol; | |
| laesst sich in ein sehr explosives Diazoniumsalz ueberfuehren,das beim Kochen mit absol.Alkohol; |
-
-
67329-16-2
4-hydroxy-3,5-dinitro-benzenesulfonic acid

-
-
573-56-8
2,6-dinitrophenol

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 180 - 200℃; im Rohr; |
-
-
64-17-5
ethanol

-
-
124-41-4
sodium methylate

-
-
606-21-3
1-chloro-2,6-dinitrobenzene

-
-
573-56-8
2,6-dinitrophenol


| Conditions | Yield |
|---|---|
| With sodium hydroxide | |
| With methanol; ethanol; sodium methylate | |
| With acetamide; sodium acetate at 170℃; | |
| Stage #1: 1-chloro-2,6-dinitrobenzene With water at 50 - 85℃; Alkaline conditions; Large scale; Stage #2: With hydrogenchloride pH=1; Large scale; |
-
-
108-95-2
phenol

-
A
-
573-56-8
2,6-dinitrophenol

-
B
-
67329-16-2
4-hydroxy-3,5-dinitro-benzenesulfonic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid Eintragen des Reaktionsgemisches in Natriumnitrat-Loesung und Zufuegen von weiterer Natriumnitrat-Loesung und konz. Schwefelsaeure unter Erwaermen auf 102-105grad; |
-
-
110-89-4
piperidine

-
-
3535-67-9
2,6-dinitroanisole

-
A
-
573-56-8
2,6-dinitrophenol

-
B
-
10156-50-0
1-piperidino-2,6-dinitrobenzene

-
C
-
20650-73-1
2,6-dinitrophenoxide ion

| Conditions | Yield |
|---|---|
| In benzene |

-
-
573-56-8
2,6-dinitrophenol

-
-
22440-82-0
2,6-diaminophenol

| Conditions | Yield |
|---|---|
| With hydrogen; palladium 10% on activated carbon In ethanol | 100% |
| With palladium 10% on activated carbon; hydrogen In methanol at 25℃; under 1050.11 Torr; for 3h; | 100% |
| With hydrogen; palladium 10% on activated carbon In methanol | 89% |

| Conditions | Yield |
|---|---|
| With hydrogen; palladium 10% on activated carbon In ethyl acetate for 5h; | 95% |
| With palladium 10% on activated carbon; hydrogen In methanol for 1h; | 92% |
| With cyclohexene; palladium on activated charcoal In ethanol at 25℃; for 16h; | 81% |

| Conditions | Yield |
|---|---|
| With iodine; triethylamine; triphenylphosphine In dichloromethane at 0 - 20℃; for 0.333333h; | 89% |
| With pyridine; thionyl chloride at 0℃; |

| Conditions | Yield |
|---|---|
| With 2,3,4,5,7,8,9,10-octahydropyrimido[1,2-a]azepin-1-ium acetate In neat (no solvent) at 50℃; for 1h; Green chemistry; | 89% |
-
-
573-56-8
2,6-dinitrophenol

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
88791-50-8
toluene-4-sulfonic acid 2,6-dinitro-phenyl ester

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane; water | 86% |
| With triethylamine In dichloromethane | 80% |
| With sodium carbonate |
-
-
61941-89-7
5-(2-chlorophenyl)-2-furoyl chloride

-
-
573-56-8
2,6-dinitrophenol

| Conditions | Yield |
|---|---|
| With PEG-400; sodium hydroxide In dichloromethane; water at 20℃; for 1h; | 82% |

| Conditions | Yield |
|---|---|
| With polyethylene glycol-400; sodium hydroxide In dichloromethane; water for 1h; Ambient temperature; | 81% |

-
-
573-56-8
2,6-dinitrophenol

-
-
149-73-5
trimethyl orthoformate

-
-
105744-50-1
di-[μ-methoxo-(2,6-dinitrophenolato)(pyridine)copper(II)]

| Conditions | Yield |
|---|---|
| With pyridine; piperidine In methanol dissolving Cu-salt and ester in abs. MeOH; keeping for 1 d at room temp.; addn. of dinitrophenol, pyridine and piperidine in MeOH; complete crystn. within 1 d; washing (MeOH); drying (vac. desiccator); elem. anal.; | 78% |


| Conditions | Yield |
|---|---|
| With pyridine for 3h; Heating; | 76% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 0 - 20℃; for 4h; Inert atmosphere; | 76% |
-
-
573-56-8
2,6-dinitrophenol

-
-
51822-12-9
4-chloroformylphenoxyacetyl chloride

| Conditions | Yield |
|---|---|
| With polyethylene glycol-400; sodium hydroxide In dichloromethane; water at 20℃; for 1h; Acylation; | 75% |
-
-
573-56-8
2,6-dinitrophenol

-
-
40466-95-3
4-bromo-2,6-dinitrophenol

| Conditions | Yield |
|---|---|
| With sulfuric acid; bromine at 100℃; for 3.5h; | 74% |
| With acetic acid beim Bromieren; | |
| With ethanol; bromine |

| Conditions | Yield |
|---|---|
| In methanol NaOH soln. added to soln. of 2,6-DNP (0.25 mmol) (pH=4-5), soln. of phen(1.0 mmol) and EuCl3*6H2O (0.5 mmol) added dropwise, stirred for 8 h; ppt. filtered off, washed with MeOH, crystd.; elem. anal.; | 72.8% |


| Conditions | Yield |
|---|---|
| In methanol NaOH soln. added to soln. of 2,6-DNP (0.25 mmol) (pH=6-7), soln. of bipy(0.25 mmol) and EuCl3*6H2O (0.5 mmol) added dropwise, stirred for 8 h; ppt. filtered off, washed with MeOH, crystd.; elem. anal.; | 72.3% |


| Conditions | Yield |
|---|---|
| In methanol NaOH soln. added to soln. of 2,6-DNP (0.25 mmol) (pH=4-5), soln. of bipy(0.25 mmol) and GdCl3*6H2O (0.5 mmol) added dropwise, stirred for 8 h; ppt. filtered off, washed with MeOH, crystd.; elem. anal.; | 71.6% |


| Conditions | Yield |
|---|---|
| 69.9% |


| Conditions | Yield |
|---|---|
| With NaOH In methanol NaOH soln. added to soln. of 2,6-DNP (0.25 mmoL) (pH=6-7), soln. of bipy(0.25 mmol) and GdCl3*6H2O (0.5 mmol) added dropwise, stirred for 8 h; ppt. filtered off, washed with MeOH, crystd.; elem. anal.; | 69.8% |


| Conditions | Yield |
|---|---|
| With NaOH In methanol NaOH soln. added to soln. of 2,6-DNP (0.25 mmol) (pH=6-7), soln. of phen(1.0 mmol) and GdCl3*6H2O (0.5 mmol) added dropwise, stirred for 8 h; ppt. filtered off, washed with MeOH, crystd.; elem. anal.; | 69.4% |

-
-
573-56-8
2,6-dinitrophenol

-
-
7205-98-3
chloromethyl phenyl sulfone

-
-
92241-31-1
3-Benzenesulfonylmethyl-2,6-dinitro-phenol

| Conditions | Yield |
|---|---|
| With sodium hydroxide In dimethyl sulfoxide at 20℃; | 69% |

| Conditions | Yield |
|---|---|
| With NaOH In methanol NaOH soln. added to soln. of 2,6-DNP (0.25 mmol) (pH=6-7), soln. of bipy(0.25 mmol) and EuCl3*6H2O (0.5 mmol) added dropwise, stirred for 8 h; ppt. filtered off, washed with MeOH, crystd.; elem. anal.; | 68.3% |


| Conditions | Yield |
|---|---|
| With NaOH In methanol NaOH soln. added to soln. of 2,6-DNP (0.25 mmol) (pH=6-7), soln. of phen(1.0 mmol) and EuCl3*6H2O (0.5 mmol) added dropwise, stirred for 8 h; ppt. filtered off, washed with MeOH, crystd.; elem. anal.; | 67.3% |


| Conditions | Yield |
|---|---|
| In methanol NaOH soln. added to soln. of 2,6-DNP (0.25 mmol) (pH=4-5), soln. of phen(1.0 mmol) and GdCl3*6H2O (0.5 mmol) added dropwise, stirred for 8 h; ppt. filtered off, washed with MeOH, crystd.; elem. anal.; | 66.8% |

Related products
Downstream Products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View