-
Name
5-Hydroxymethylpyrrolidin-2-one
- EINECS -0
- CAS No. 66673-40-3
- Article Data24
- CAS DataBase
- Density 1.153 g/cm3
- Solubility
- Melting Point 83-85 °C(lit.)
- Formula C5H9NO2
- Boiling Point 346 °C at 760 mmHg
- Molecular Weight 115.132
- Flash Point 163 °C
- Transport Information
- Appearance White powder
- Safety 26-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 2-Pyrrolidinone,5-(hydroxymethyl)-, (R)-;(-)-5-(Hydroxymethyl)-2-pyrrolidinone;(5R)-5-Hydroxymethylpyrrolidin-2-one;(R)-(-)-5-(Hydroxymethyl)-2-pyrrolidinone;(R)-5-(Hydroxymethyl)pyrrolidin-2-one;(R)-5-Hydroxymethyl-2-pyrrolidinone;(R)-5-Hydroxymethyl-2-pyrrolidone;(R)-5-Hydroxymethylpyrrolidin-2-one;(R)-5-Oxopyrrolidine-2-methanol;(R)-(-)-5-hydroxymethyl-2-pyrrolidinone;
- PSA 49.33000
- LogP -0.41390
Synthetic route

-
-
66673-40-3
(R)-5-hydroxymethylpyrrolidin-2-one

| Conditions | Yield |
|---|---|
| With water; trifluoroacetic acid In dichloromethane at 50℃; for 5h; | 99% |
-
-
64700-65-8
methyl (2R)-pyroglutamate

-
-
66673-40-3
(R)-5-hydroxymethylpyrrolidin-2-one

| Conditions | Yield |
|---|---|
| With methanol; sodium tetrahydroborate at 0℃; | 97% |
| With methanol; sodium tetrahydroborate at 0℃; | 97% |
| With methanol; sodium tetrahydroborate at 0℃; | 97% |
-
-
68766-96-1
ethyl (R)-2-pyrrolidone-5-carboxylate

-
-
66673-40-3
(R)-5-hydroxymethylpyrrolidin-2-one

| Conditions | Yield |
|---|---|
| With lithium borohydride In tetrahydrofuran for 16h; Ambient temperature; | 85% |
| With sodium tetrahydroborate In tetrahydrofuran; methanol at 5℃; for 1h; | 80% |
| With sodium tetrahydroborate In ethanol for 2h; Ambient temperature; | 79% |
-
-
4042-36-8
D-pyrrolidone-5-carboxylic acid

-
-
66673-40-3
(R)-5-hydroxymethylpyrrolidin-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 94 percent / SOCl2 / 1.5 h / 80 °C 2: 85 percent / LiBH4 / tetrahydrofuran / 16 h / Ambient temperature View Scheme | |
| Multi-step reaction with 2 steps 1: 95 percent / thionyl chloride / 1.) 0 deg C, 30 min, 2.) room temp., 3 h 2: 78 percent / NaBH4 / ethanol / 2 h / Ambient temperature View Scheme | |
| Multi-step reaction with 2 steps 1: SOCl2 / 48 h / Ambient temperature; pH=0 2: 58 percent / NaBH4 / propan-2-ol / 20 h / Ambient temperature View Scheme |
-
-
6893-26-1
D-Glutamic acid

-
-
66673-40-3
(R)-5-hydroxymethylpyrrolidin-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 1) thionyl chloride / 1a) room temp., 1 h, 1b) reflux, 30 min. 2: 32.5 g / 1.5 h / 150 °C / 15 Torr 3: 79 percent / sodium borohydride / ethanol / 2 h / Ambient temperature View Scheme | |
| Multi-step reaction with 2 steps 1: 88 percent / SOCl2, KOH / 150 °C 2: LiBH4 View Scheme | |
| Multi-step reaction with 3 steps 1: H2O / 63 h / Heating 2: SOCl2 / 48 h / Ambient temperature; pH=0 3: 58 percent / NaBH4 / propan-2-ol / 20 h / Ambient temperature View Scheme | |
| Multi-step reaction with 2 steps 1.1: thionyl chloride 1.2: Amberlyst A-21 1.3: Reflux 2.1: sodium tetrahydroborate / isopropyl alcohol View Scheme | |
| Multi-step reaction with 2 steps 1.1: thionyl chloride / 20 h / 20 °C 1.2: 2 h / Reflux 2.1: sodium tetrahydroborate / isopropyl alcohol / 20 h / 20 °C View Scheme |
-
-
13107-55-6
D-glutamic acid diethyl ester

-
-
66673-40-3
(R)-5-hydroxymethylpyrrolidin-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 32.5 g / 1.5 h / 150 °C / 15 Torr 2: 79 percent / sodium borohydride / ethanol / 2 h / Ambient temperature View Scheme |
-
-
13515-99-6, 23150-65-4, 27025-25-8
D-glutamic acid dimethyl ester hydrochloride

-
-
66673-40-3
(R)-5-hydroxymethylpyrrolidin-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: toluene / 4 h / Reflux 2: isopropyl alcohol; sodium tetrahydroborate / 20 h / 20 °C View Scheme |
-
-
66673-40-3
(R)-5-hydroxymethylpyrrolidin-2-one

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
128899-30-9, 100548-49-0, 106191-02-0
(R)-5-(((tert-butyldimethylsilyl)oxy)methyl)pyrrolidin-2-one

| Conditions | Yield |
|---|---|
| With 1H-imidazole In dichloromethane at 20℃; for 4h; | 100% |
| With 1H-imidazole In N,N-dimethyl-formamide for 24h; Ambient temperature; | 99% |
| With 1H-imidazole In N,N-dimethyl-formamide at 20℃; | 99% |
-
-
66673-40-3
(R)-5-hydroxymethylpyrrolidin-2-one

-
-
386707-15-9
methyl 3'-(cyanocarbonyl)-2,2'-binaphthalene-3-carboxylate

| Conditions | Yield |
|---|---|
| With dmap In acetonitrile at 14℃; for 1.5h; | 100% |
-
-
66673-40-3
(R)-5-hydroxymethylpyrrolidin-2-one

-
-
77-76-9
2,2-dimethoxy-propane

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene for 1.5h; Dean-Stark; Reflux; | 100% |
| With camphor-10-sulfonic acid at 75℃; for 20h; Reagent/catalyst; Temperature; Time; Concentration; | 78% |
| With camphor-10-sulfonic acid at 75℃; for 20h; Time; Temperature; Reflux; | 78% |
-
-
66673-40-3
(R)-5-hydroxymethylpyrrolidin-2-one

-
-
109-92-2
ethyl vinyl ether

-
-
67040-46-4
(5R)-5-((tert-ethoxyethoxy)methyl)pyrrolidin-2-one

| Conditions | Yield |
|---|---|
| With trichloroacetic acid In chloroform for 4h; Ambient temperature; | 97% |
| With trichloroacetic acid In dichloromethane at 20℃; for 16h; | 37% |
| With trichloroacetic acid In dichloromethane at 20℃; for 16h; | 37% |
| With trichloroacetic acid In dichloromethane at 20℃; for 16h; | 37% |
| With trichlorosilane; acetic acid In dichloromethane at 20℃; for 16h; | 37% |
-
-
66673-40-3
(R)-5-hydroxymethylpyrrolidin-2-one

-
-
4042-36-8
D-pyrrolidone-5-carboxylic acid

| Conditions | Yield |
|---|---|
| With oxygen; sodium hydrogencarbonate; platinum In water for 4h; Ambient temperature; | 95% |
-
-
66673-40-3
(R)-5-hydroxymethylpyrrolidin-2-one

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
128899-31-0
<(2R)-5-oxopyrrolidin-2-yl>methyl 4-methylbenzenesulfonate

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane at 20℃; | 95% |
| With dmap; triethylamine In dichloromethane at 0 - 23℃; for 12h; Inert atmosphere; | 93% |
| With dmap; triethylamine In dichloromethane at 20℃; for 18h; Cooling with ice; | 81% |
-
-
66673-40-3
(R)-5-hydroxymethylpyrrolidin-2-one

-
-
58479-61-1
tert-butylchlorodiphenylsilane

-
-
138629-44-4
(R)-5-(tert-butyl-diphenyl-silanyloxymethyl)-pyrrolidin-2-one

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide for 12h; Ambient temperature; | 95% |
| With 1H-imidazole In N,N-dimethyl-formamide at 20℃; for 5h; | 95% |
| With dmap; triethylamine In dichloromethane | 88% |
-
-
66673-40-3
(R)-5-hydroxymethylpyrrolidin-2-one

-
-
124-63-0
methanesulfonyl chloride

-
-
128811-47-2
<(2R)-5-oxopyrrolidin-2-yl>methyl methanesulfonate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0℃; for 1.5h; | 93% |
| With triethylamine In dichloromethane | 85% |
| With triethylamine In chloroform at 0 - 20℃; | 70% |
| With pyridine at 25℃; for 1.5h; | 60% |

| Conditions | Yield |
|---|---|
| With potassium carbonate; copper(l) iodide; N,N`-dimethylethylenediamine In acetonitrile for 18h; Heating / reflux; | 93% |
-
-
13154-24-0
triisopropylsilyl chloride

-
-
66673-40-3
(R)-5-hydroxymethylpyrrolidin-2-one

| Conditions | Yield |
|---|---|
| With 1H-imidazole In dichloromethane; N,N-dimethyl-formamide at 20℃; Inert atmosphere; | 88% |
| With 1H-imidazole In dichloromethane at 0 - 20℃; for 16h; | 37% |
-
-
66673-40-3
(R)-5-hydroxymethylpyrrolidin-2-one

-
-
776-74-9
Bromodiphenylmethane

| Conditions | Yield |
|---|---|
| Stage #1: (R)-5-hydroxymethylpyrrolidin-2-one With sodium hydride In tetrahydrofuran; mineral oil at 0℃; for 0.5h; Inert atmosphere; Stage #2: Bromodiphenylmethane In tetrahydrofuran; mineral oil at 50℃; for 12h; | 88% |
-
-
66673-40-3
(R)-5-hydroxymethylpyrrolidin-2-one

-
-
173828-76-7
(R)-5-(azidomethyl)-2-pyrrolidone

| Conditions | Yield |
|---|---|
| With hydrogen azide; di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 20℃; for 1h; | 87% |
| With tris-(2-chloro-ethyl)-amine; triphenylphosphine; diethylazodicarboxylate |
-
-
66673-40-3
(R)-5-hydroxymethylpyrrolidin-2-one

-
-
100-52-7
benzaldehyde

-
-
118918-76-6
(2S,5R)-2-phenyl-1-aza-3-oxabicyclo<3.3.0>octan-8-one

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene Reflux; Dean-Stark; | 85% |
| With 4 A molecular sieve; toluene-4-sulfonic acid In toluene Heating; | 72% |
| With toluene-4-sulfonic acid Inert atmosphere; | 67% |
| With water; toluene-4-sulfonic acid In toluene at 20℃; Reflux; | 62% |
| With toluene-4-sulfonic acid In toluene for 16h; Reflux; | 52% |
-
-
2362-12-1
4-Bromo-2-methylphenol

-
-
66673-40-3
(R)-5-hydroxymethylpyrrolidin-2-one

-
-
215382-97-1
(R)-5-(4-Bromo-2-methylphenoxymethyl)-2-pyrrolidinone

| Conditions | Yield |
|---|---|
| In ethyl acetate; SiO2 | 83% |
-
-
66673-40-3
(R)-5-hydroxymethylpyrrolidin-2-one

-
-
836643-56-2
[1-(4-bromo-phenyl)-hexyloxy]-tert-butyl-dimethyl-silane

-
-
1073241-71-0
C23H39NO3Si

| Conditions | Yield |
|---|---|
| With copper(l) iodide; potassium carbonate; N,N`-dimethylethylenediamine In acetonitrile Heating / reflux; | 82% |

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid In dichloromethane at 20℃; for 6h; Inert atmosphere; | 80% |
-
-
5470-17-7
3-bromo-2-chloro-5-nitropyridine

-
-
66673-40-3
(R)-5-hydroxymethylpyrrolidin-2-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 100℃; for 16h; Inert atmosphere; | 79% |
-
-
66673-40-3
(R)-5-hydroxymethylpyrrolidin-2-one

-
-
98612-60-3
(5R)-5-(bromomethyl)pyrrolidin-2-one

| Conditions | Yield |
|---|---|
| With carbon tetrabromide; triphenylphosphine In dichloromethane for 1.5h; Ambient temperature; | 77% |
| With carbon tetrabromide; triphenylphosphine In acetonitrile | 73% |
| With carbon tetrabromide; triphenylphosphine In acetonitrile at 0 - 20℃; for 24h; |
-
-
98491-29-3
1-iodo-4-(methoxymethoxy)benzene

-
-
66673-40-3
(R)-5-hydroxymethylpyrrolidin-2-one

| Conditions | Yield |
|---|---|
| With copper(l) iodide; caesium carbonate; N,N`-dimethylethylenediamine In N,N-dimethyl-formamide Heating; | 71% |
-
-
215388-44-6
3-methoxy-4-[2-(1-pyrrolidinyl)ethoxy]phenyl 2-(4-hydroxyphenyl)benzo[b]thiophen-3-yl ketone

-
-
66673-40-3
(R)-5-hydroxymethylpyrrolidin-2-one

-
-
215387-32-9
3-Methoxy-4-[2-(1-pyrrolidinyl)ethoxy]phenyl (R)-2-[4-(5-Oxopyrrolidin-2-ylmethoxy)phenyl]benzo[b]thiophen-3-yl Ketone

| Conditions | Yield |
|---|---|
| With triethanolamine In tetrahydrofuran; SiO2 | 70% |
-
-
27149-27-5
2-(4-bromophenyl)-1,3-thiazole

-
-
66673-40-3
(R)-5-hydroxymethylpyrrolidin-2-one

-
-
1073242-22-4
C14H14N2O2S

| Conditions | Yield |
|---|---|
| With copper(l) iodide; potassium carbonate; N,N`-dimethylethylenediamine In acetonitrile for 18h; Heating / reflux; | 67% |

| Conditions | Yield |
|---|---|
| With potassium carbonate; copper(l) iodide; N,N`-dimethylethylenediamine In acetonitrile for 18h; Heating / reflux; | 67% |
-
-
66673-40-3
(R)-5-hydroxymethylpyrrolidin-2-one

-
-
138541-53-4
(R)-(+)-5-(chloromethyl)-2-pyrrolidone

| Conditions | Yield |
|---|---|
| With methanesulfonyl chloride In N,N-dimethyl-formamide at 65℃; for 16h; | 65% |
-
-
23703-22-2
1-bromo-4-n-hexylbenzene

-
-
66673-40-3
(R)-5-hydroxymethylpyrrolidin-2-one

-
-
1043876-44-3
C17H25NO2

| Conditions | Yield |
|---|---|
| With copper(l) iodide; potassium carbonate; N,N`-dimethylethylenediamine In acetonitrile for 72h; Heating/reflux; | 63% |
| With potassium carbonate; copper(l) iodide; N,N`-dimethylethylenediamine In acetonitrile for 72h; Reflux; | 63% |
-
-
106-43-4
para-chlorotoluene

-
-
66673-40-3
(R)-5-hydroxymethylpyrrolidin-2-one

| Conditions | Yield |
|---|---|
| With copper(I) oxide; potassium phosphate; N1,N2-bis(thiophen-2-ylmethyl)oxalamide In tert-butyl alcohol at 130℃; for 24h; Schlenk technique; | 58% |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
66673-40-3
(R)-5-hydroxymethylpyrrolidin-2-one

-
-
58479-61-1
tert-butylchlorodiphenylsilane

-
-
149-73-5
trimethyl orthoformate

-
-
203301-82-0
(5R)-1-(tert-butoxycarbonyl)-2-methoxy-5-(tert-butyldiphenylsilanyloxymethyl)pyrrolidine

| Conditions | Yield |
|---|---|
| Multistep reaction.; | 52% |

-
-
193966-79-9
6-benzyloxy-2-(4-hydroxyphenyl)-3-[3-methoxy-4-[(1-pyrrolidinyl)methyl]benzyl]benzo[b]thiophene

-
-
66673-40-3
(R)-5-hydroxymethylpyrrolidin-2-one

-
-
215388-70-8
(R)-6-Benzyloxy-3-[3-methoxy-4-(1-pyrrolidinylmethyl)benzyl]-2-[4-(5-oxopyrrolidin-2-ylmethoxy)phenyl]benzo[b]thiophene

| Conditions | Yield |
|---|---|
| 50% |
(R)-(-)-5-Hydroxymethylpyrrolidin-2-one Chemical Properties
Synonyms: (R)-5-HYDROXYMETHYL-PYRROLIDIN-2-ONE;(R)-(-)-5-(HYDROXYMETHYL)-2-PYRROLIDINONE;(R)-5-(HYDROXYMETHYL)-2-PYRROLIDINONE; D-PYROGLUTAMINOL;(-)-D-PYROGLUTAMOL;(R)-(-)-5-(HYDROXYMETHYL)-2-PYRROLIDONE;(R)-(-)-2-PYRROLIDONE-5-YL-METHANOL;
The Molecular formula of 5-Hydroxymethylpyrrolidin-2-one(66673-40-3): C5H9NO2
The Molecular Weight of 5-Hydroxymethylpyrrolidin-2-one(66673-40-3): 115.13
Molecular Structure :
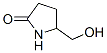
Surface Tension: 38.7 dyne/cm
Density: 1.153 g/cm3
Flash Point: 163 °C
Enthalpy of Vaporization: 68.34 kJ/mol
Boiling Point: 346 °C at 760 mmHg
Vapour Pressure: 3.72E-06 mmHg at 25°C
Appearance: White Powder
(R)-(-)-5-Hydroxymethylpyrrolidin-2-one Safety Profile
 Xi
Xi The Risk Statements information of 5-Hydroxymethylpyrrolidin-2-one(66673-40-3):
36/37/38: Irritating to eyes, respiratory system and skin
The Safety Statements information of 5-Hydroxymethylpyrrolidin-2-one(66673-40-3):
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36: Wear suitable protective clothing
WGK Germany: 3
F: 3-10
Related Products
- (R)-(-)-5-Hydroxymethylpyrrolidin-2-one
- 66674-16-6
- 666746-27-6
- 666747-06-4
- 6667-60-3
- 6668-24-2
- 666839-74-3
- 66684-57-9
- 66684-58-0
- 66684-59-1
- 66684-60-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View