-
Name
1'-Acetonaphthone
- EINECS 213-384-1
- CAS No. 941-98-0
- Article Data265
- CAS DataBase
- Density 1.098 g/cm3
- Solubility immiscible with water
- Melting Point 10-12 °C
- Formula C12H10O
- Boiling Point 296.999 °C at 760 mmHg
- Molecular Weight 170.211
- Flash Point 129.458 °C
- Transport Information
- Appearance clear pale yellow to yellow liquid
- Safety 26-36
- Risk Codes 36/37/38-22
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi,  Xn
Xn
- Synonyms 1'-Acetonaphthone(6CI,8CI);1-(1-Naphthalenyl)ethanone;1-(1-Naphthyl)ethanone;1-(Naphthalen-4-yl)ethanone;1-Acetonaphthalene;1-Acetonaphthone;1-Acetylnaphthalene;1-Naphthyl methyl ketone;Methyl 1-naphthyl ketone;Methyla-naphthyl ketone;NSC 7659;a-Acetonaphthone;a-Acetylnaphthalene;a-Naphthyl methyl ketone;
- PSA 17.07000
- LogP 3.04240
Synthetic route
-
-
57605-95-5
1-(1-naphthyl)ethanol

-
-
941-98-0
1'-naphthacetophenone

| Conditions | Yield |
|---|---|
| With ruthenium trichloride; iodobenzene; potassium peroxomonosulfate In water; acetonitrile at 20℃; for 7h; | 100% |
| With ruthenium trichloride; iodobenzene; potassium peroxymonosulfate In water; acetonitrile at 20℃; for 7h; Inert atmosphere; | 100% |
| With silica gel supported bis(trimethylsilyl) chromate In dichloromethane at 25℃; for 0.333333h; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: methyllithium; 1-Cyanonaphthalene In tetrahydrofuran at -90℃; for 0.5h; Stage #2: With water | 100% |
-
-
91-20-3
naphthalene

-
-
75-36-5
acetyl chloride

-
A
-
941-98-0
1'-naphthacetophenone

-
B
-
93-08-3
methyl 2-naphthyl ketone

| Conditions | Yield |
|---|---|
| aluminium trichloride In 1,2-dichloro-ethane at 0℃; Rate constant; Kinetics; Mechanism; other temperature; other concentrations; | A 99% B 1% |
| aluminium trichloride In 1,2-dichloro-ethane at 0℃; Thermodynamic data; ΔH<*>(activation); Σ<*>(activation); | A 99% B 1% |
| With aluminium trichloride; 1-ethyl-3-methyl-1H-imidazol-3-ium chloride at 0℃; for 1h; | A 89% B 2% |

-
-
102421-44-3
4-methyl-N'-(1-(naphthalen-1-yl)ethylidene)benzenesulfonohydrazide

-
-
941-98-0
1'-naphthacetophenone

| Conditions | Yield |
|---|---|
| With silica-supported selenamide; dihydrogen peroxide In tert-butyl alcohol at 55℃; for 20h; | 99% |

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid; water; palladium diacetate; 2-(3,5-dimethyl-1H-pyrazol-1-yl)pyridine at 60℃; for 5h; | 98% |

| Conditions | Yield |
|---|---|
| With triethylamine; Pd(Ac2O)2-DPPP In N,N-dimethyl-formamide at 80℃; for 0.5h; | 97% |
| With triethylamine; Pd(Ac2O)2-DPPP In N,N-dimethyl-formamide at 80℃; for 0.5h; reaction with other aryl triflates; | 97% |
| With hydrogenchloride; 1,3-bis-(diphenylphosphino)propane; triethylamine; palladium diacetate 1) DMF, 80 deg C, 1 h; Yield given. Multistep reaction; | |
| With hydrogenchloride; triethylamine; palladium diacetate; 2.9-dimethyl-1,10-phenanthroline 1.) DMF, 40 deg C, 2.5 h, 2.) 0.5 h; Yield given. Multistep reaction; |
-
-
15727-65-8
1-ethynylnaphthalene

-
-
941-98-0
1'-naphthacetophenone

| Conditions | Yield |
|---|---|
| With cobalt(III)((CH2NCHC6H4(O))2)(OAc); sulfuric acid In methanol; water at 80℃; for 20h; Schlenk technique; | 97% |
| With sulfuric acid; C18H15CoN2O10S2(2-)*2Na(1+); water In methanol at 80℃; for 20h; Schlenk technique; | 96% |
| With methanol; [Co((dimethylglyoximate)BF2)2•2H2O] at 65℃; for 2.5h; Sealed tube; Neutral conditions; regioselective reaction; | 93% |

| Conditions | Yield |
|---|---|
| Stage #1: -butyl vinyl ether; 1-Bromonaphthalene With palladium diacetate; 1,3-bis-(diphenylphosphino)propane; triethylamine In various solvent(s) at 100℃; for 24h; Heck reaction; Stage #2: With hydrogenchloride Further stages.; | 95% |
| Stage #1: -butyl vinyl ether; 1-Bromonaphthalene With meso-2,4-bis(diphenylphosphino)pentane; triethylamine; palladium diacetate In dimethyl sulfoxide at 115℃; for 36h; Heck arylation; Stage #2: With hydrogenchloride In dimethyl sulfoxide at 20℃; | 94% |
| Stage #1: -butyl vinyl ether; 1-Bromonaphthalene With triethylamine; tris-(o-tolyl)phosphine; 3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate; 1,3-bis-(diphenylphosphino)propane; palladium dichloride at 130℃; for 2h; Heck reaction; microwave irradiation; Stage #2: With hydrogenchloride In water Further stages.; | 71% |
| With hydrogenchloride; triethylamine; palladium diacetate; 2.9-dimethyl-1,10-phenanthroline 1.) DMF, 80 deg C, 24 h, 2.) 0.5 h; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| Stage #1: naphthalene With copper(I) oxide In acetonitrile at 25℃; for 0.666667h; Stage #2: formyl acetic anhydride With oxalic acid In acetonitrile for 2h; Temperature; | 95% |

-
-
941-98-0
1'-naphthacetophenone

| Conditions | Yield |
|---|---|
| With potassium fluoride; dihydrogen peroxide; potassium hydrogencarbonate In tetrahydrofuran; methanol; water at 60℃; for 10h; Tamao-Kumada Oxidation; Schlenk technique; Glovebox; | 94% |
-
-
1956-40-7, 100485-51-6, 100485-59-4
1-(1-naphthalenyl)ethanone oxime

-
-
941-98-0
1'-naphthacetophenone

| Conditions | Yield |
|---|---|
| With potassium permanganate; montmorillonite K-10 for 0.333333h; | 93% |
| With bismuth(III) nitrate; silica gel In tetrahydrofuran; water for 9h; Heating; | 87% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In toluene 1.) 0 deg C, 3 min, then 15 deg C, 5 min, 2.) -10 deg C; | 92% |
-
-
15914-84-8
(S)-1-(1-Naphthyl)ethanol

-
-
941-98-0
1'-naphthacetophenone

| Conditions | Yield |
|---|---|
| With 2-azaadamantane-N-oxyl; oxygen In aq. acetate buffer at 20℃; for 8h; pH=4.5; Reagent/catalyst; Green chemistry; Enzymatic reaction; chemoselective reaction; | 92% |
-
-
24168-42-1
2-(naphthalen-1-yl)propanenitrile

-
-
941-98-0
1'-naphthacetophenone

| Conditions | Yield |
|---|---|
| With sodium hydroxide; N-benzyl-N,N,N-triethylammonium chloride; oxygen In dimethyl sulfoxide for 3h; | 91% |

-
-
941-98-0
1'-naphthacetophenone

| Conditions | Yield |
|---|---|
| With 2,2,2-trifluoroethanol; oxygen; vanadium(V) oxychloride for 3h; deprotection; Heating; | 91% |
| With 2,2,6,6-tetramethylpiperidinyl-lithium In tetrahydrofuran; hexane at -78℃; for 4h; Inert atmosphere; | 88% |
| With methanol; sodium tetrahydroborate; nickel(II) chloride hexahydrate at 20℃; for 0.75h; | 88% |
-
-
139214-46-3
2-methyl-2-(naphthalen-1-yl)-1,3-dithiane

-
-
941-98-0
1'-naphthacetophenone

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; tantalum pentachloride; sodium iodide In water; ethyl acetate at 20℃; for 2.5h; | 91% |
| With dihydrogen peroxide; tantalum pentachloride; sodium iodide In water; ethyl acetate at 20℃; for 2.5h; | 91% |
| With Amberlite IR-120; palladium on activated charcoal In methanol for 18h; Heating; | 81% |
-
-
3385-78-2
trimethyl indium

-
-
879-18-5
naphthalene-1-carbonic acid chloride

-
-
941-98-0
1'-naphthacetophenone

| Conditions | Yield |
|---|---|
| With mesoporous MCM-41-immobilized phosphine-free heterogeneous palladium(0)-schiff base complex In tetrahydrofuran at 68℃; for 2h; Inert atmosphere; Green chemistry; | 91% |

| Conditions | Yield |
|---|---|
| With 2C60H80NaO12(2+)*Cl6Pd2(2-); potassium hydrogencarbonate; triphenylphosphine In toluene at 110℃; for 3h; Inert atmosphere; | 89% |

| Conditions | Yield |
|---|---|
| With [2,2]bipyridinyl; palladium diacetate; trifluoroacetic acid In tetrahydrofuran; water at 80℃; for 36h; Schlenk technique; Inert atmosphere; | 87% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-(2-hydroxy-1-naphthyl)ethan-1-one With TentaGel-N(R)C(O)CH2C2F4OC2F4SO2F; potassium carbonate In N,N-dimethyl-formamide at 20℃; Stage #2: With formic acid; 1,3-bis-(diphenylphosphino)propane; triethylamine; palladium diacetate In N,N-dimethyl-formamide at 85℃; for 2h; Further stages.; | 86% |
-
-
457634-29-6
trimethyl-(1-naphthalen-1-yl-ethoxy)-silane

-
-
941-98-0
1'-naphthacetophenone

| Conditions | Yield |
|---|---|
| With γ-picolinium chlorochromate In dichloromethane at 20℃; for 6h; | 85% |

| Conditions | Yield |
|---|---|
| With 1,3-bis-(diphenylphosphino)propane; palladium diacetate; p-benzoquinone In N,N-dimethyl-formamide at 130℃; for 0.0833333h; oxidative Heck reaction; regiospecific reaction; | 85% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; diethyl ether; hexane at 20℃; for 15h; Solvent; Schlenk technique; Inert atmosphere; chemoselective reaction; | 85% |
-
-
26870-27-9
1-(naphthalen-1-yl)-2-phenoxyethan-1-one

-
-
941-98-0
1'-naphthacetophenone

| Conditions | Yield |
|---|---|
| With 2,6-bis[1-(2,6-dimethylphenylimino)ethyl]pyridine cobalt(II)dichloride; bis(pinacol)diborane; sodium t-butanolate In tetrahydrofuran; methanol at 65℃; for 3h; Schlenk technique; Inert atmosphere; | 84% |
-
-
201230-82-2
carbon monoxide

-
-
99747-74-7
1-naphthyl triflate

-
-
594-27-4
tetramethylstannane

-
-
941-98-0
1'-naphthacetophenone

| Conditions | Yield |
|---|---|
| With 2,6-di-tert-butyl-4-methyl-phenol; 4 A molecular sieve; lithium chloride; (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride In N,N-dimethyl-formamide at 90℃; under 2660 Torr; for 1.5h; | 83% |


| Conditions | Yield |
|---|---|
| With LiCl; CO; dichloro{1,1'-bis(diphenylphosphino)ferrocene}palladium(II) In N,N-dimethyl-formamide High Pressure; the triflate and the stannane in DMF contg. the Pd catalyst and LiCl heated at 90°C under high pressure CO for 1,5 h; cooled to room temp., dild. with ether, filtered, filtrate washed with water and satd. NaCl soln., dried, concd., chromd. (flash column, hexanes/EtOAc); | 83% |
-
-
201230-82-2
carbon monoxide

-
-
90-14-2
1-Iodonaphthalene

-
-
3385-78-2
trimethyl indium

-
-
941-98-0
1'-naphthacetophenone

| Conditions | Yield |
|---|---|
| With mesoporous material MCM-41-immobilized palladium(II)-schiff base complex In tetrahydrofuran at 68℃; under 760.051 Torr; for 3h; Inert atmosphere; | 83% |

-
-
913827-31-3
1-acetyl-1,4-dihydro-1,4-epoxynaphthalene

-
-
941-98-0
1'-naphthacetophenone

| Conditions | Yield |
|---|---|
| With triphenyl phosphite; perrhenic acid anhydride In toluene at 100℃; for 18h; Inert atmosphere; | 83% |
-
-
1402034-43-8
4,4,5,5-tetramethyl-2-(1-(naphthalen-1-yl)ethyl)-1,3,2-dioxaborolane

-
-
941-98-0
1'-naphthacetophenone

| Conditions | Yield |
|---|---|
| With 2-(2-methoxyphenylamino)-4-(2-methoxyphenylimino)pent-2-ene; copper diacetate In pyridine; acetic acid; acetonitrile at 60℃; for 16h; chemoselective reaction; | 82% |

| Conditions | Yield |
|---|---|
| With tert.-butylnitrite; N-hydroxyphthalimide In acetonitrile at 80℃; for 24h; Schlenk technique; | 80% |
| With tert.-butylhydroperoxide; 1-n-butyl-3-methylimidazolim bromide In water at 20℃; for 12h; | 80% |
| With FeH6Mo6O24(3-)*3H3N*3H(1+)*7H2O; tetrabutylammomium bromide; dihydrogen peroxide In 1,4-dioxane at 70℃; for 24h; | 78% |
-
-
941-98-0
1'-naphthacetophenone

-
-
57605-95-5
1-(1-naphthyl)ethanol

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In ethanol for 2h; | 100% |
| Stage #1: 1'-naphthacetophenone With sodium tetrahydroborate In ethanol Stage #2: With hydrogenchloride In ethanol | 100% |
| Stage #1: 1'-naphthacetophenone With Trimethyl borate; dimethylsulfide borane complex; (-)-3-amino-6,6-dimethyl-2-hydroxy-bicyclo[3.1.1]heptane In tetrahydrofuran at 20℃; Stage #2: With methanol In tetrahydrofuran | 98% |
-
-
941-98-0
1'-naphthacetophenone

-
-
15914-84-8
(S)-1-(1-Naphthyl)ethanol

| Conditions | Yield |
|---|---|
| With [(R,R,R)-Ru(BINAP-PO3H2)(DPEN)Cl2] on magnetite nanoparticle; potassium tert-butylate; hydrogen In isopropyl alcohol at 20℃; under 36200.4 Torr; for 20h; | 100% |
| With potassium hydroxide; (R,R)-1,2-diphenylethylenediamine; hydrogen; [RuCl2((R)-3,3'-dm-binap)(dmf)n] In isopropyl alcohol at 20℃; under 6080 Torr; | 99% |
| With bis(1,5-cyclooctadiene)diiridium(I) dichloride; C53H73FeN2O2PS; hydrogen; lithium tert-butoxide In isopropyl alcohol at 60℃; under 22801.5 Torr; for 12h; Autoclave; enantioselective reaction; | 99% |
-
-
941-98-0
1'-naphthacetophenone

-
-
78-08-0
Triethoxyvinylsilane

-
-
161467-57-8
2'-<2-(triethoxysilyl)ethyl>-1'-acetonaphthone

| Conditions | Yield |
|---|---|
| With [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; sodium formate; triphenylphosphine In toluene at 140℃; for 0.3h; | 100% |
| With carbonyl bis(hydrido)tris(triphenylphosphine)ruthenium(II) In toluene for 6h; Heating; | 67% |
| With bis(1,5-cyclooctadiene)iridium(I) tetrakis[3,5-bis(trifluoromethyl)phenyl]borate; C38H28P2 In 1,3-dioxane at 130℃; for 24h; Reagent/catalyst; regioselective reaction; | 45% |
-
-
941-98-0
1'-naphthacetophenone

-
-
72824-04-5
2-Allyl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane

-
-
745813-65-4, 869541-47-9
2-(naphthalen-1-yl)pent-4-en-2-ol

| Conditions | Yield |
|---|---|
| With indium iodide In tetrahydrofuran at 40℃; for 24h; | 100% |
| With methanol; indium (III) tris(hexamethyldisilyl) amide In toluene at 20℃; for 6h; Inert atmosphere; | 100% |
| With (PPh3)3CuF In tetrahydrofuran at 20℃; for 3h; | 98% |
| With indium In water at 30℃; for 24h; Inert atmosphere; | 87% |

| Conditions | Yield |
|---|---|
| With sodium at 0 - 20℃; for 3h; Claisen Condensation; | 100% |
-
-
941-98-0
1'-naphthacetophenone

-
-
42177-25-3
(R)-1-(naphth-1-yl)ethanol

| Conditions | Yield |
|---|---|
| With potassium hydroxide; hydrogen; (R)-3,3'-dimethyl-[1,1'-binaphthalene]-2,2'-diamine; [RuCl2((R)-3,3'-dm-binap)(dmf)n]; (S,S)-1,2-diphenyl-1,2-diaminoethane In isopropyl alcohol at 20℃; under 6080 Torr; for 4h; | 99% |
| With [Fe(II)(N-isocyano-N-isopropylpropan-2-amine)2((5S,8S,14aS,18aS)-5,8-diphenyl-5,6,7,8,13,14,14a,15,16,17,18,18a,19,20-tetradecahydrotribenzo[b,f,l][1,4]diaza[8,11]diphosphacyclotetradecine)](BF4)2; isopropyl alcohol; sodium t-butanolate at 75℃; for 1.5h; Glovebox; enantioselective reaction; | 99.3% |
| With bis(1,5-cyclooctadiene)diiridium(I) dichloride; (SC,SC,RFC)-1-(diphenylphosphino)-2-[1-N-((4S)-4-tert-butyl-2-oxazolinyl-2-ylmethyl)ethyl]ferrocene; hydrogen; potassium carbonate In isopropyl alcohol at 25℃; under 15201 Torr; for 2h; Glovebox; Autoclave; enantioselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol; water at 20℃; Claisen Schmidt condensation; Cooling with ice; | 99.2% |

| Conditions | Yield |
|---|---|
| With potassium fluoride; 4-chloromethoxybenzene; palladium diacetate In tetrahydrofuran; water at 20℃; for 1h; Inert atmosphere; chemoselective reaction; | 99% |
| With polymethylhydrosiloxane; iron(III) chloride hexahydrate In 1,2-dichloro-ethane at 120℃; for 1h; Microwave irradiation; | 98% |
| With triethylsilane; indium(III) bromide In chloroform at 60℃; for 1h; Inert atmosphere; | 95% |
-
-
941-98-0
1'-naphthacetophenone

-
-
13686-51-6
2-bromoaceto-1-naphthone

| Conditions | Yield |
|---|---|
| With pyridinium hydrobromide perbromide In tetrahydrofuran; chloroform at 20℃; for 18h; | 99% |
| With bromine In 1,4-dioxane at 20℃; for 0.833333h; | 93% |
| With pyridinium hydrobromide perbromide In tetrahydrofuran; chloroform at 20℃; | 93.5% |
-
-
941-98-0
1'-naphthacetophenone

-
-
24824-93-9
chloromethyl p-tolyl sulfoxide

| Conditions | Yield |
|---|---|
| With n-butyllithium; diisopropylamine In tetrahydrofuran at -65℃; for 0.416667h; | 99% |
-
-
941-98-0
1'-naphthacetophenone

-
A
-
15914-84-8
(S)-1-(1-Naphthyl)ethanol

-
B
-
42177-25-3
(R)-1-(naphth-1-yl)ethanol

| Conditions | Yield |
|---|---|
| With dimethylsulfide borane complex; (S)-2-(anilinodiphenylmethyl)pyrrolidine In tetrahydrofuran at 20℃; for 2h; enantioselective reaction; | A n/a B 99% |
| With dimethylsulfide borane complex In tetrahydrofuran at 20℃; for 2h; enantioselective reaction; | A n/a B 99% |
| With (mer-[(S,S)-1,5-dimethyl-2,4-bis(4-phenyl-1,3-oxazolin-2-yl)benzene(1-)]Ru(CO)Cl)2(ZnCl2); hydrogen; sodium methylate In isopropyl alcohol at 40℃; under 22801.5 Torr; for 24h; Inert atmosphere; Autoclave; optical yield given as %ee; enantioselective reaction; | A 96% B n/a |

| Conditions | Yield |
|---|---|
| With C84H110N10Zn2 In neat (no solvent) at 20℃; for 12h; Sealed tube; Inert atmosphere; Schlenk technique; Glovebox; | 99% |
| chloro(1,5-cyclooctadiene)rhodium(I) dimer; asymmetric tetraphenyl-tetraoxa-phosphazulene derivative In toluene at 0℃; for 24h; enantioselective hydrosilylation; | |
| With silver tetrafluoroborate; (S)-[RhBr(nbd)(oxazolinyl-carbene)] In dichloromethane at -60℃; for 10h; |

| Conditions | Yield |
|---|---|
| With C29H46LaN3Si2 at 15℃; for 2h; Inert atmosphere; Glovebox; Schlenk technique; | 99% |
| With zinc(II) iodide In acetonitrile for 48h; Addition; | 97% |
| With antimony(III) chloride at 20℃; for 0.25h; Neat (no solvent); | 95% |
-
-
941-98-0
1'-naphthacetophenone

-
-
95046-87-0
C6H4C4H3COC(2)H3

| Conditions | Yield |
|---|---|
| With water-d2 In Dimethoxymethane | 99% |
| With water-d2; sodium hydroxide |
-
-
941-98-0
1'-naphthacetophenone

-
-
39684-28-1
O-(tert-butyl)hydroxylamine hydrochloride

-
-
1177266-16-8
1-acetylnaphthalene O-tert-butyl oxime

| Conditions | Yield |
|---|---|
| With sodium acetate In ethanol at 20℃; for 27h; | 99% |
-
-
941-98-0
1'-naphthacetophenone

-
-
141-97-9
ethyl acetoacetate

-
-
109-77-3
malononitrile

-
-
1235990-62-1
6-amino-2,4-dihydro-3,4-dimethyl-4-(naphthalen-1-yl)pyrano[2,3-c]pyrazole-5-carbonitrile

| Conditions | Yield |
|---|---|
| With hydrazine hydrate; heptakis(6-amino-6-deoxy)-β-cyclodextrin at 20℃; for 0.0166667h; Neat (no solvent); | 99% |


| Conditions | Yield |
|---|---|
| With [Cp*Co(vinyltrimethylsilane)2] In benzene-d6 at 20℃; for 14h; Catalytic behavior; Schlenk technique; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With C84H110N10Zn2 In neat (no solvent) at 20℃; for 6h; Sealed tube; Inert atmosphere; Schlenk technique; Glovebox; | 99% |
| With C14H24N4Si(1+)*I(1-) In benzene-d6 at 90℃; for 6h; Catalytic behavior; Inert atmosphere; Schlenk technique; regioselective reaction; | 86% |
| With bis-(phosphoranyl)methanido aluminum hydride In benzene-d6 at 110℃; for 6h; Inert atmosphere; | 54% |
| With C27H44AlN3 at 20℃; for 4h; Inert atmosphere; Glovebox; |

| Conditions | Yield |
|---|---|
| With C114H137F6N3O8P2S2 In diethyl ether at -20℃; Mukaiyama Aldol Addition; enantioselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: 1'-naphthacetophenone With bis(trifluoromethanesulfonyl)amide In diethyl ether at -78 - 23℃; Mukaiyama Aldol Addition; Schlenk technique; Inert atmosphere; Stage #2: ketene t-butyldimethylsilyl methyl acetal In diethyl ether at -20℃; for 0.5h; Mukaiyama Aldol Addition; Schlenk technique; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With bis(1,5-cyclooctadiene)iridium(I) tetrakis[3,5-bis(trifluoromethyl)phenyl]borate; C38H28P2 In 1,3-dioxane at 130℃; for 24h; Reagent/catalyst; regioselective reaction; | 99% |
-
-
941-98-0
1'-naphthacetophenone

-
-
1576-35-8
toluene-4-sulfonic acid hydrazide

-
-
102421-44-3
4-methyl-N'-(1-(naphthalen-1-yl)ethylidene)benzenesulfonohydrazide

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol; water for 24h; Reflux; | 98% |
| In methanol at 20℃; | 89% |
| With hydrogenchloride In ethanol for 1h; Heating; | 77% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol at 50 - 60℃; Sonication; Green chemistry; | 98% |


| Conditions | Yield |
|---|---|
| With methanol; O-benzenesulfonyl-acetohydroxamic acid ethyl ester; toluene-4-sulfonic acid at 23℃; for 24h; Inert atmosphere; | 98% |
| With O-benzenesulfonyl-acetohydroxamic acid ethyl ester; toluene-4-sulfonic acid In ethanol at 20℃; for 24h; | 62% |
-
-
941-98-0
1'-naphthacetophenone

-
-
917-64-6
methyl magnesium iodide

-
-
6301-54-8
2-(naphthalen-1-yl)propan-2-ol

| Conditions | Yield |
|---|---|
| In diethyl ether for 2h; Ambient temperature; | 97.7% |
1'-Acetonaphthone Chemical Properties
Product Name: 1'-Acetonaphthone (CAS NO.941-98-0)
Molecular Formula:C12H10O
Molar mass:170.2072 g/mol
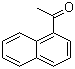
Form: powder
Density: 1.097 g/cm3
Flash Point: 129.5 °C
Boiling Point: 297 °C at 760 mmHg
Index of Refraction: 1.615
Vapour Pressure: 0.00139 mmHg at 25°C
Melting point: 10-12°C
Water solubility: immiscible
Enthalpy of Vaporization: 53.68 kJ/mol
Index of Refraction: 1.615
Molar Refractivity: 54.12 cm3
Molar Volume: 155 cm3
XLogP3-AA: 2.9
H-Bond Donor: 0
H-Bond Acceptor: 1
Structure Descriptors of 1'-Acetonaphthone (CAS NO.941-98-0):
IUPAC Name: 1-naphthalen-1-ylethanone
Canonical SMILES: CC(=O)C1=CC=CC2=CC=CC=C21
InChI: InChI=1S/C12H10O/c1-9(13)11-8-4-6-10-5-2-3-7-12(10)11/h2-8H,1H3
InChIKey: QQLIGMASAVJVON-UHFFFAOYSA-N
Product Categories: Aromatic Ketones (substituted)
1'-Acetonaphthone Uses
1'-Acetonaphthone (CAS NO.941-98-0) can be used for intermediates of dye and pharmaceutical.
1'-Acetonaphthone Production
1'-Acetonaphthone (CAS NO.941-98-0) has been derived by acetylation of naphthalene with acetic anhydride in the presence of aluminum chloride.
1'-Acetonaphthone Toxicity Data With Reference
| 1. | skn-rbt 500 mg/24H MLD | FCTOD7 Food and Chemical Toxicology. 20 (1982),755. | ||
| 2. | orl-rat LD50:1560 mg/kg | FCTOD7 Food and Chemical Toxicology. 20 (1982),755. |
1'-Acetonaphthone Consensus Reports
Reported in EPA TSCA Inventory.
1'-Acetonaphthone Safety Profile
Moderately toxic by ingestion. A skin irritant. A combustible liquid. When heated to decomposition it emits acrid smoke and irritating fumes.
Safety Information of 1'-Acetonaphthone (CAS NO.941-98-0):
Hazard Codes:Xi ,Xn
,Xn
Risk Statements:
22: Harmful if swallowed
36: Irritating to the eyes
37: Irritating to the respiratory system
38: Irritating to the skin
Safety Statements:
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36: Wear suitable protective clothing
RTECS: BD1200000
F: 3
Hazard Note: Irritant
HS Code: 29143900
1'-Acetonaphthone Standards and Recommendations
DOT Classification: 3; Label: Flammable Liquid
1'-Acetonaphthone Specification
1'-Acetonaphthone , its CAS NO. is 941-98-0, the synonyms are 1-Acetylnaphthalene ; 1-Acetonaphthone ; alpha-Acetonaphthone ; alpha-Acetylnaphthalene ; 1-Acetonaphthalene ; 1-Naphthyl methyl ketone ; .alpha.-Acetonaphthone ; alpha-Naphthyl methyl ketone ; Methyl 1-naphthyl ketone ; 1-(1-naphthalenyl)-Ethanone ; 1-(1-naphthalenyl)-ethanon .
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View