-
Name
1,3,5-Trimethoxybenzene
- EINECS 210-673-4
- CAS No. 621-23-8
- Article Data114
- CAS DataBase
- Density 1.041 g/cm3
- Solubility insoluble in water
- Melting Point 50-53 °C(lit.)
- Formula C9H12O3
- Boiling Point 257 °C at 760 mmHg
- Molecular Weight 168.192
- Flash Point 85.6 °C
- Transport Information UN 1325
- Appearance white to cream crystalline powder
- Safety 24/25-36
- Risk Codes 22-20/21/22
-
Molecular Structure
-
Hazard Symbols
 Xn;
Xn;  F
F
- Synonyms 4-06-00-07362 (Beilstein Handbook Reference);Phloroglucinol trimethyl ether;Benzene, 1,3,5-trimethoxy-;1,3,5-Trimethoxybenzene (JAN);O,O,O-1,3,5-Trimethylresorcinol;
- PSA 27.69000
- LogP 1.71240
Synthetic route

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); tert-butyl XPhos In N,N-dimethyl-formamide at 100℃; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: methanol; 1,3,5-trisbromobenzene In toluene at 135℃; under 5320.36 Torr; for 0.75h; Stage #2: With triethylamine In toluene at 165℃; under 8360.56 Torr; for 11h; Temperature; | 99.5% |
| With sodium methylate; copper(l) chloride In N,N-dimethyl-formamide at 100 - 110℃; for 6h; | 90% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 3h; Reflux; | 99% |
| With potassium carbonate In acetone for 6h; Reflux; | 80% |
| With methanol man troepfelt methylalkoholische Natriummethylatloesung hinzu; |

| Conditions | Yield |
|---|---|
| With palladium(II) trifluoroacetate; trifluoroacetic acid In dimethyl sulfoxide; N,N-dimethyl-formamide at 70℃; for 24h; | 99% |
| With [Rh(OH)(cod)]2; 1,3-bis-(diphenylphosphino)propane; water; sodium hydroxide In toluene at 120℃; for 18h; Inert atmosphere; | 85% |
| With sodium persulfate; ethanol at 60℃; for 18h; Temperature; Time; Sealed tube; Green chemistry; | 85% |
| With C40H42NO7PPdS In dichloromethane at 20℃; Schlenk technique; |

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In water at 80℃; for 0.5h; Diels-Alder Cycloaddition; | A n/a B 99% |
-
-
35564-11-5
1,3,5-trimethoxybenzene-2,4,6-d3

-
-
621-23-8
1,2,3-trimethoxybenzene

| Conditions | Yield |
|---|---|
| With tris(pentafluorophenyl)borate; water In chloroform at 80℃; for 24h; Sealed tube; | 98% |
| With acetic acid In hexane at 24.9℃; Kinetics; Thermodynamic data; Rate constant; other solvents; ΔH(activ.); -ΔS(activ.); ΔF(activ.).; |

| Conditions | Yield |
|---|---|
| With methanol; Methyl formate; copper(l) chloride at 115℃; for 2h; Autoclave; Green chemistry; | 98% |
| With copper(I) chloride In N,N-dimethyl-formamide at 130℃; for 6h; | 91% |

-
A
-
621-23-8
1,2,3-trimethoxybenzene

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In water at 80℃; for 0.5h; Diels-Alder Cycloaddition; | A n/a B 98% |

-
A
-
621-23-8
1,2,3-trimethoxybenzene

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In water at 80℃; for 0.5h; Diels-Alder Cycloaddition; | A n/a B 97% |
-
-
36086-05-2
2-trimethylsilyl-1,3,5-trimethoxybenzene

-
-
621-23-8
1,2,3-trimethoxybenzene

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane; water; potassium iodide In acetonitrile at 20℃; for 0.1h; | 96% |

-
-
621-23-8
1,2,3-trimethoxybenzene

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid at 120℃; | 96% |

| Conditions | Yield |
|---|---|
| With N,N'-dimethylimidazolium-2-carboxylate In acetonitrile at 160℃; for 1.33333h; Microwave irradiation; Green chemistry; | 96% |

| Conditions | Yield |
|---|---|
| With sodium; copper(I) iodide In N-methyl-acetamide; methanol | 95% |
-
-
861389-87-9
1,3,5-trimethoxy-2-(buta-1,3-dienyl)benzene

-
-
621-23-8
1,2,3-trimethoxybenzene

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid at 120℃; | 95% |
-
-
67827-53-6
ethyl 3-(2,4,6-trimethoxy phenyl)acrylate

-
-
621-23-8
1,2,3-trimethoxybenzene

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid at 120℃; | 95% |

| Conditions | Yield |
|---|---|
| rhodium(III) chloride; 1,3-bis-(diphenylphosphino)propane In diethylene glycol dimethyl ether for 40h; Heating; | 94% |
| With hydrogen In cyclohexane at 165℃; under 15001.5 - 26252.6 Torr; for 4h; Solvent; Autoclave; | 90.9% |
| With bis(1,5-cyclooctadiene)diiridium(I) dichloride; air; triphenylphosphine In 1,4-dioxane for 48h; Heating; | 78% |
| With methanol; scandium tris(trifluoromethanesulfonate) Mechanism; other aromatic aldehydes; |

-
-
621-23-8
1,2,3-trimethoxybenzene

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid at 120℃; | 94% |

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In water at 80℃; for 0.5h; Solvent; Temperature; Time; Diels-Alder Cycloaddition; | A n/a B 94% |

-
A
-
621-23-8
1,2,3-trimethoxybenzene

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In water at 80℃; for 0.5h; Diels-Alder Cycloaddition; | A n/a B 93% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone | 92% |
| With potassium carbonate In acetone for 48h; Reflux; | 92% |
| With potassium carbonate In acetone for 0.0666667h; Heating; Microwave irradiation; | 91% |
-
-
40243-91-2
1,3,5-trimethoxy 2-vinyl benzene

-
-
621-23-8
1,2,3-trimethoxybenzene

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid at 120℃; | 92% |

-
A
-
621-23-8
1,2,3-trimethoxybenzene

-
B
-
132725-78-1
7-methyl-2-(4-methylphenyl)naphthalene

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In water at 80℃; for 0.5h; Diels-Alder Cycloaddition; | A n/a B 92% |

-
A
-
621-23-8
1,2,3-trimethoxybenzene

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In water at 80℃; for 0.5h; Diels-Alder Cycloaddition; | A n/a B 92% |

-
A
-
621-23-8
1,2,3-trimethoxybenzene

-
B
-
38251-86-4
2-(4'-bromophenyl)-7-bromonaphthalene

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In water at 80℃; for 0.5h; Diels-Alder Cycloaddition; | A n/a B 92% |

-
-
621-23-8
1,2,3-trimethoxybenzene

| Conditions | Yield |
|---|---|
| With Octanethiol; trifluorormethanesulfonic acid In dichloromethane at 23℃; for 12h; | 91% |

| Conditions | Yield |
|---|---|
| With tin(IV) chloride In 1,2-dichloro-ethane Heating; | 90% |

-
-
621-23-8
1,2,3-trimethoxybenzene

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid at 120℃; | 90% |

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In water at 80℃; for 0.5h; Diels-Alder Cycloaddition; | A n/a B 90% |

-
A
-
621-23-8
1,2,3-trimethoxybenzene

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In water at 80℃; for 0.5h; Diels-Alder Cycloaddition; | A n/a B 90% |

-
A
-
621-23-8
1,2,3-trimethoxybenzene

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In water at 80℃; for 0.5h; Diels-Alder Cycloaddition; | A n/a B 90% |

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In tetrachloromethane for 3h; Heating; | 100% |
| With ammonium metavanadate; oxygen; aluminium bromide In diethyl ether at 20℃; for 18h; | 99% |
| With perchloric acid; C34H45MoNO5; tetrabutylammomium bromide; dihydrogen peroxide In d7-N,N-dimethylformamide at 28℃; for 0.4h; | 99% |

| Conditions | Yield |
|---|---|
| With ammonium metavanadate; oxygen; aluminium bromide In 1,4-dioxane at 80℃; for 4h; | 100% |
| With hydrogen bromide; dimethyl sulfoxide In water; ethyl acetate at 60℃; for 2h; | 94% |
| With oxygen; lithium bromide; copper(ll) bromide In acetic acid at 60℃; under 760.051 Torr; regioselective reaction; | 93% |
-
-
621-23-8
1,2,3-trimethoxybenzene

| Conditions | Yield |
|---|---|
| With dinitrogen tetraoxide; Nitrogen dioxide In dichloromethane Nitration; | 100% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; tin(IV) chloride In benzene | 100% |
-
-
621-23-8
1,2,3-trimethoxybenzene

-
-
1928-76-3
9-benzyl-6-chloro-9H-purine

-
-
1241556-48-8
6-(1,3,5-trimethoxyphen-4-yl)-9-phenylmethyl-9H-purine

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid; 1,1,1,3',3',3'-hexafluoro-propanol at 60℃; for 24h; | 100% |
| With aluminum (III) chloride In 1,2-dichloro-ethane for 0.5h; Reflux; | 95% |
-
-
621-23-8
1,2,3-trimethoxybenzene

-
-
1323902-49-3
tert-butyl ((ethylthio)(phenyl)methyl)carbamate

-
-
1373935-52-4
tert-butyl N-[phenyl(2,4,6-trimethoxyphenyl)methyl]carbamate

| Conditions | Yield |
|---|---|
| With N-iodo-succinimide In dichloromethane at -78℃; for 0.0833333h; Solvent; Temperature; Reagent/catalyst; Friedel-Crafts Alkylation; Sealed tube; Inert atmosphere; chemoselective reaction; | 100% |

-
-
621-23-8
1,2,3-trimethoxybenzene

| Conditions | Yield |
|---|---|
| With iron(III) chloride In dichloromethane at 20℃; for 0.166667h; Inert atmosphere; regioselective reaction; | 100% |

| Conditions | Yield |
|---|---|
| With iron(III) chloride In dichloromethane at 20℃; for 0.166667h; Inert atmosphere; regioselective reaction; | 100% |
-
-
621-23-8
1,2,3-trimethoxybenzene

-
-
132145-64-3
dimethyl 2-(p-methylphenyl)cyclopropane-1,1-dicarboxylate

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid at 20℃; for 3h; | 100% |
| With ytterbium(III) triflate In 1,2-dichloro-ethane at 20 - 65℃; Catalytic behavior; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| In 2,2,2-trifluoroethanol at 0 - 20℃; for 1h; Inert atmosphere; | 100% |

-
-
621-23-8
1,2,3-trimethoxybenzene

-
-
1110700-49-6
dimethyl 2-(2-fluorophenyl)cyclopropane-1,1-dicarboxylate

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid at 40℃; for 4h; | 100% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In 1,2-dichloro-ethane at 100℃; for 4h; Sealed tube; | 100% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In 1,2-dichloro-ethane at 100℃; for 4h; Sealed tube; | 100% |
-
-
621-23-8
1,2,3-trimethoxybenzene

-
-
84655-87-8
SS-morpholino 4-toluene(dithioperoxo)sulfonate

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In dichloromethane at 20℃; for 16h; Inert atmosphere; | 100% |
| Multi-step reaction with 2 steps 1: trifluoroacetic acid; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical / dichloromethane / 1 h / 20 °C / Inert atmosphere 2: trifluoroacetic acid / dichloromethane / 16 h / 20 °C / Inert atmosphere View Scheme |
-
-
621-23-8
1,2,3-trimethoxybenzene

-
-
78-94-4
methyl vinyl ketone

-
-
53581-92-3
4-(2,4,6-trimethoxy-phenyl)-butan-2-one

| Conditions | Yield |
|---|---|
| gold(III) chloride; silver trifluoromethanesulfonate In 1,2-dichloro-ethane at 20℃; | 99% |
| gold(III) chloride In acetonitrile at 20℃; for 24h; | 99.5% |
| With boron trifluoride diethyl etherate In dichloromethane at 20℃; for 0.0333333h; Michael Addition; Green chemistry; | 93% |
| With dichloro bis(acetonitrile) palladium(II); tin(ll) chloride In acetonitrile at 30℃; for 2h; Kinetics; Reagent/catalyst; Time; Solvent; Michael Addition; Inert atmosphere; Schlenk technique; | 92% |
| With boron trifluoride diethyl etherate |
-
-
621-23-8
1,2,3-trimethoxybenzene

-
-
25603-67-2
9-phenyl-fluoren-9-ol

-
-
115322-67-3
9-phenyl-9-(2,4,6-trimethoxyphenyl)-9H-fluorene

| Conditions | Yield |
|---|---|
| With scandium tris(trifluoromethanesulfonate) In dichloromethane at 60℃; for 4h; Sealed tube; | 99% |
| With perchloric acid; nitromethane |
-
-
621-23-8
1,2,3-trimethoxybenzene

-
-
105404-90-8
1,3,5-tribromo-2,4,6-trimethoxybenzene

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In acetonitrile at 20℃; for 144h; | 99% |
| With bromine excess of reagent; | 80% |
| With bromine; iron(III) chloride In 1,2-dichloro-ethane Heating; | 70% |
| With bromine |
-
-
621-23-8
1,2,3-trimethoxybenzene

-
-
100-52-7
benzaldehyde

-
-
54921-79-8
2,2'-(phenylmethylene)bis(1,3,5-trimethoxybenzene)

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane; bismuth(III) sulfate at 20℃; for 1h; | 99% |
| With boron trifluoride diethyl etherate In dichloromethane at 20℃; for 0.0833333h; Friedel-Crafts Alkylation; | 96% |
| With PEG-400 at 50 - 60℃; for 2h; Friedel-Crafts Alkylation; Green chemistry; | 92% |
-
-
621-23-8
1,2,3-trimethoxybenzene

-
-
75-36-5
acetyl chloride

-
-
832-58-6
1-(2,4,6-trimethoxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| Stage #1: 1,2,3-trimethoxybenzene In dichloromethane at 0℃; for 0.166667h; Stage #2: acetyl chloride With aluminum (III) chloride In dichloromethane for 2h; Friedel-Crafts Acylation; | 99% |
| With zinc(II) chloride | 98% |
| With 1,1,1,3',3',3'-hexafluoro-propanol at 20℃; for 5h; Friedel-Crafts Acylation; Inert atmosphere; | 92% |
-
-
621-23-8
1,2,3-trimethoxybenzene

-
-
125343-69-3
2,3,4,6-tetra-O-benzyl-1-O-(trifluoroacetyl)-α-D-glucopyranose

-
-
82300-67-2
1-deoxy-1-(C-2',4',6'-trimethoxyphenyl) 2,3,4,6-tetra-O-benzyl-β-D-glucopyranoside

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate In dichloromethane for 0.0833333h; Ambient temperature; | 99% |
| With boron trifluoride diethyl etherate In dichloromethane for 0.0833333h; Product distribution; Ambient temperature; var. Lewis acids and aryl ethers;; | 99% |

| Conditions | Yield |
|---|---|
| With orcinol; Acetobacterium dehalogenans veratrol-O-demethylase; Desulfitobacterium hafniense methyltransferase dhaf4611 In aq. buffer at 25℃; for 24h; pH=6.5; Inert atmosphere; Enzymatic reaction; regioselective reaction; | 99% |
| With N,N,N,N,N,N-hexamethylphosphoric triamide; sodium hydride; N-methylaniline In diethyl ether; xylene at 95℃; for 9h; | 85% |
| With 2-(diethylamino)ethanethiol hydrochloride; sodium t-butanolate In N,N-dimethyl-formamide for 2h; Heating; | 84% |
-
-
621-23-8
1,2,3-trimethoxybenzene

-
-
108-24-7
acetic anhydride

-
-
832-58-6
1-(2,4,6-trimethoxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With scandium tris(trifluoromethanesulfonate) In acetonitrile for 0.5h; Time; Friedel-Crafts Acylation; Inert atmosphere; Microwave irradiation; Heating; Green chemistry; | 99% |
| With trifluoroacetic acid at 20℃; for 1.5h; Friedel-Crafts Acylation; | 95% |
| With boron trifluoride diethyl etherate In ethyl acetate at 20℃; for 2h; Friedel-Crafts Acylation; | 93% |
-
-
621-23-8
1,2,3-trimethoxybenzene

-
-
555-16-8
4-nitrobenzaldehdye

-
-
54921-80-1
(4-nitrophenyl)-bis-(2,4,6-trimethoxyphenyl)methane

| Conditions | Yield |
|---|---|
| With tin(IV) chloride; bis(1,5-cyclooctadiene)diiridium(I) dichloride at 90℃; for 1h; | 99% |
| With air; iodine In toluene at 60℃; for 24h; Friedel Crafts alkylation; | 93% |
| With [1,2,3,4,5-pentamethylcyclopentadiene Ir(SnCl3)2{SnCl2(H2O)2}] In 1,2-dichloro-ethane at 80℃; for 1h; | 90% |
-
-
621-23-8
1,2,3-trimethoxybenzene

-
-
194720-38-2
2,3,5,6-tetramethyl-4-oxo-1-phenylthiocyclohexa-2,5-dienyl 2-chloroacetate

-
A
-
41280-62-0
1,3,5-trimethoxy-2-(phenylsulfanyl)benzene

| Conditions | Yield |
|---|---|
| With trimethylsilyl trifluoromethanesulfonate In acetonitrile for 0.166667h; Yields of byproduct given; | A 99% B n/a |

-
-
621-23-8
1,2,3-trimethoxybenzene

-
-
194720-38-2
2,3,5,6-tetramethyl-4-oxo-1-phenylthiocyclohexa-2,5-dienyl 2-chloroacetate

-
-
41280-62-0
1,3,5-trimethoxy-2-(phenylsulfanyl)benzene

| Conditions | Yield |
|---|---|
| With trimethylsilyl trifluoromethanesulfonate In acetonitrile at -30 - 20℃; for 0.166667h; | 99% |
-
-
621-23-8
1,2,3-trimethoxybenzene

-
-
335642-28-9
1-(2,3,4,5,6-pentafluorophenylthio)-2,3,5,6-tetramethyl-4-oxocyclohexa-2,5-dienyl 2-chloroacetate

| Conditions | Yield |
|---|---|
| With trimethylsilyl trifluoromethanesulfonate In acetonitrile at -30 - 0℃; for 0.166667h; | A n/a B 99% |


| Conditions | Yield |
|---|---|
| With aluminium trichloride In 1,2-dichloro-ethane at 75℃; for 3.5h; Friedel-Crafts reaction; | 99% |
1,3,5-Trimethoxybenzene Consensus Reports
1,3,5-Trimethoxybenzene Specification
1. Introduction of 1,3,5-Trimethoxybenzene
The IUPAC name of this chemical is 1,3,5-Trimethoxybenzene. With the CAS registry number 621-23-8 and EINECS 210-673-4, it is also named as Benzene,1,3,5-trimethoxy-. In addition, the formula is C9H12O3 and the molecular weight is 168.19. It belongs to the classes of Aromatic Ethers; Active Pharmaceutical Ingredients; Benzene derivates. And it is a kind of white crystalline powder.
2. Properties of 1,3,5-Trimethoxybenzene
Physical properties about 1,3,5-Trimethoxybenzene are: (1)ACD/LogP: 1.61; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.61; (4)ACD/LogD (pH 7.4): 1.61; (5)#H bond acceptors: 3; (6)#H bond donors: 0; (7)#Freely Rotating Bonds: 3; (8)Polar Surface Area: 27.69 Å2; (9)Index of Refraction: 1.485; (10)Molar Refractivity: 46.28 cm3; (11)Molar Volume: 161.4 cm3; (12)Polarizability: 18.35 ×10-24cm3; (13)Surface Tension: 29.8 dyne/cm; (14)Density: 1.041 g/cm3; (15)Flash Point: 85.6 °C; (16)Enthalpy of Vaporization: 47.46 kJ/mol; (17)Boiling Point: 257 °C at 760 mmHg; (18)Vapour Pressure: 0.0239 mmHg at 25°C.
3. Structure Descriptors of 1,3,5-Trimethoxybenzene
(1)SMILES: COc1cc(cc(OC)c1)OC
(2)InChI: InChI=1/C9H12O3/c1-10-7-4-8(11-2)6-9(5-7)12-3/h4-6H,1-3H3
(3)InChIKey: LKUDPHPHKOZXCD-UHFFFAOYAM
4. Toxicity of 1,3,5-Trimethoxybenzene
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 580mg/kg (580mg/kg) | Oyo Yakuri. Pharmacometrics. Vol. 3, Pg. 187, 1969. | |
| mouse | LD50 | oral | 1480mg/kg (1480mg/kg) | Oyo Yakuri. Pharmacometrics. Vol. 3, Pg. 187, 1969. | |
| mouse | LD50 | subcutaneous | 2800mg/kg (2800mg/kg) | Oyo Yakuri. Pharmacometrics. Vol. 3, Pg. 187, 1969. |
5. Use of 1,3,5-Trimethoxybenzene
1,3,5-Trimethoxybenzene it can be used to get 2-bromo-1,3,5-trimethoxy-benzene. This reaction will need reagents 30percent H2O2, aq. HClO4 and KBr, catalyst ammonium metavanadate and solvents methanol and H2O. The reaction time is 24 hours at ambient temperature. The yield is about 90%.

6. Preparation of 1,3,5-Trimethoxybenzene
1,3,5-Trimethoxybenzene can be prepared by sulfuric acid dimethyl ester and benzene-1,3,5-triol. This reaction will need reagent K2CO3 and solvent acetone. The reaction time is 24 hours and the yield is about 80% by heating.
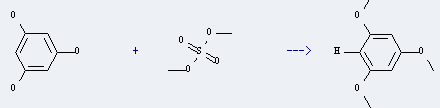
7. Other details of 1,3,5-Trimethoxybenzene
When you are using this chemical, please be cautious about it as the following:
It is harmful by inhalation, in contact with skin and if swallowed. You should avoid contact with skin and eyes and wear suitable protective clothing when you are using it.
Related Products
- 10,10'-Bis([1,1'-biphenyl]-4-yl)-9,9'-bianthracene
- 10,10'-Dibromo-9,9'-bianthryl
- 10,10-Dimethylanthrone
- 10,10-Oxybisphenoxarsine
- 10-[1,1'-Biphenyl]-4-yl-2-(1-methylethyl)-9-oxo-9H-thioxanthenium hexafluorophosphate
- 10,11-Dehydroimipramine
- 10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine
- 10,11-Dihydro-2-(4-methyl-1-piperazinyl)-11-(2-athiazolyl)-pyridazino(3,4-b)(1,4)benzoxazepine
- 10,11-DIHYDRO-2-(4-METHYL-1-PIPERAZINYL)-11-(3,4-XYLYL)PYRIDAZINO(3,4b)(1,4)-BENZOXAZEPINE MALEATE
- 10,11-Dihydro-5-(3-dimethylamino-2-methylpropyl)-5h-dibenz (b,f)azepine
- 62123-80-2
- 62124-27-0
- 62124-43-0
- 62125-22-8
- 621-27-2
- 62127-47-3
- 62129-39-9
- 621-29-4
- 62129-44-6
- 62129-53-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View