-
Name
1,3-Bis(2,6-diisopropylphenyl)imidazolium chloride
- EINECS 627-434-9
- CAS No. 250285-32-6
- Article Data50
- CAS DataBase
- Density
- Solubility Slightly soluble in water.
- Melting Point 278 °C (dec.)(lit.)
- Formula C27H37ClN2
- Boiling Point
- Molecular Weight 425.057
- Flash Point
- Transport Information
- Appearance White, off-white or tan powder
- Safety 26-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 1H-Imidazolium,1,3-bis[2,6-bis(1-methylethyl)phenyl]-, chloride (9CI);1,3-Bis(2,6-diisopropylphenyl)-1,3-dihydro-2H-imidazol-2-ylidenemonohydrochloride;1,3-Bis(2,6-diisopropylphenyl)-1H-imidazolium chloride;1,3-Bis[2,6-bis(1-methylethyl)phenyl]-1H-imidazolium chloride;1,3-(2,6-Diisopropylphenyl)imidazolium Chloride;
- PSA 8.81000
- LogP 4.25160
Synthetic route
-
-
1207373-09-8
C34H50Cl2N4Pd

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In chloroform-d1 at 20℃; for 12h; Inert atmosphere; | 100% |
-
-
50-00-0
formaldehyd

-
-
74663-75-5
1,2-bis(2,6-diisopropylphenylimino)ethane

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane In ethyl acetate at 70℃; for 2h; | 97% |
| With chloro-trimethyl-silane In ethyl acetate at 70℃; for 2h; Inert atmosphere; Glovebox; | 87% |
| With chloro-trimethyl-silane In ethyl acetate at 70℃; for 2.75h; Inert atmosphere; | 86% |
-
-
74663-75-5
1,2-bis(2,6-diisopropylphenylimino)ethane

-
-
3188-13-4
ethyl chloromethyl ether

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 24h; Molecular sieve; Inert atmosphere; | 94% |
| With air In tetrahydrofuran; water at 40℃; for 16h; Solvent; | 65% |
| With water In tetrahydrofuran at 40℃; for 16h; Condensation; | 47% |
-
-
50-00-0
formaldehyd

-
-
74663-75-5
(1E,2E)-N1,N2-bis(2,6-diisopropylphenyl)-ethane-1,2-diimine

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane In ethyl acetate at 70℃; for 2h; | 90% |
| With chloro-trimethyl-silane In ethyl acetate at 70℃; for 2h; | 89% |
| With hydrogenchloride In 1,4-dioxane; ethyl acetate at 20℃; for 17h; | 86% |
| Stage #1: formaldehyd; (1E,2E)-N1,N2-bis(2,6-diisopropylphenyl)-ethane-1,2-diimine In toluene at 100℃; Formylation; Stage #2: With hydrogenchloride In 1,4-dioxane; toluene at 70℃; for 5h; chloridation; | 47% |
| With hydrogenchloride In 1,4-dioxane; ethyl acetate at 20℃; Heating; | 41% |
-
-
50-00-0
formaldehyd

-
-
131543-46-9
Glyoxal

-
-
24544-04-5
2,6-diisopropylbenzenamine

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol for 36h; Reflux; | 84.6% |

-
-
578743-87-0
chloro[1,3-bis(2,6-di-i-propylphenyl)imidazol-2-ylidene]copper(I)

-
A
-
1060651-05-9
1,3-bis[2,6-di(propan-2-yl)phenyl]-1,3-dihydro-2H-imidazol-2-one

-
B
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| With air In dimethylsulfoxide-d6 at 150℃; for 155h; | A 16% B 81% |
| With water; oxygen In dimethylsulfoxide-d6 at 150℃; for 155h; Kinetics; Sealed tube; | A 16 %Spectr. B 81 %Spectr. |
-
-
67-56-1
methanol

-
-
74663-75-5
1,2-bis(2,6-diisopropylphenylimino)ethane

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| Stage #1: methanol; 1,2-bis(2,6-diisopropylphenylimino)ethane With sodium tetrahydroborate In tetrahydrofuran at 0 - 20℃; for 3h; Stage #2: With ammonium chloride In tetrahydrofuran | 80% |
-
-
244187-81-3
1,3-bis-(2,6-diisopropylphenyl)-imidazol-2-ylidene

-
B
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| With trichlorosilane In toluene at 25℃; for 12h; Inert atmosphere; | A 79% B n/a |
-
-
74663-75-5
1,2-bis(2,6-diisopropylphenylimino)ethane

-
-
123-63-7
paracetaldehyde

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane In ethyl acetate at 70℃; for 2.75h; | 76% |
-
-
50-00-0
formaldehyd

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In tetrahydrofuran; 1,4-dioxane at 40℃; for 72h; | 58% |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 140℃; for 20h; Inert atmosphere; Schlenk technique; Sealed tube; | A n/a B 54% |

-
-
107-30-2
chloromethyl methyl ether

-
-
74663-75-5
1,2-bis(2,6-diisopropylphenylimino)ethane

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| at 40℃; for 16h; Sealed tube; | 52% |
| at 40℃; for 16h; Sealed tube; | 52% |
-
-
24544-04-5
2,6-diisopropylbenzenamine

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: 77.5 percent / formic acid / ethanol; H2O / 48 h 2.1: toluene / 100 °C 2.2: 47 percent / HCl / toluene; dioxane / 5 h / 70 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 90 percent / propan-1-ol; H2O / 1 h / 70 °C 2: 47 percent / water / tetrahydrofuran / 16 h / 40 °C View Scheme | |
| Multi-step reaction with 2 steps 1: ethanol / 12 h / 20 °C 2: tetrahydrofuran / 18 h / 40 °C / Inert atmosphere View Scheme |
-
-
131543-46-9
Glyoxal

-
-
24544-04-5
2,6-diisopropylbenzenamine

-
-
3188-13-4
ethyl chloromethyl ether

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| Inert atmosphere; |


-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| Stage #1: trans-[PdCl2(1,3-diethylimidazole)(1,3-(2,6-(i-Pr)2C6H3)2C3H2N2)] With silver tetrafluoroborate In [D3]acetonitrile at 80℃; for 48h; Inert atmosphere; Stage #2: With lithium chloride In [D3]acetonitrile Inert atmosphere; |
-
-
1080030-15-4
C27H36Cl3N2P

-
-
244187-81-3
1,3-bis-(2,6-diisopropylphenyl)-imidazol-2-ylidene

-
B
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| at -30 - 20℃; for 50h; Inert atmosphere; |


-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| With chloroform |
-
-
25364-47-0
1-(2,6-diisopropylphenyl)-1H-imidazole

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| In acetonitrile for 36h; Reflux; Inert atmosphere; Schlenk technique; |
-
-
131543-46-9
Glyoxal

-
-
24544-04-5
2,6-diisopropylbenzenamine

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane In formic acid |

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 140℃; for 21h; Inert atmosphere; Schlenk technique; Sealed tube; | 21 %Chromat. |

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 140℃; for 21h; Inert atmosphere; Schlenk technique; Sealed tube; | 18 %Chromat. |

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 100℃; for 1h; Inert atmosphere; Schlenk technique; Sealed tube; | A 49 %Chromat. B 41 %Chromat. |
| In N,N-dimethyl-formamide at 140℃; for 21h; Inert atmosphere; Schlenk technique; Sealed tube; | A 32 %Chromat. B 56 %Chromat. |
| Multi-step reaction with 2 steps 1: chloroform-d1 / 24 h / 40 °C / Inert atmosphere; Schlenk technique; Sealed tube 2: N,N-dimethyl-formamide / 1 h / 100 °C / Inert atmosphere; Schlenk technique; Sealed tube View Scheme |

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: N,N-dimethyl-formamide / 21 h / 140 °C / Inert atmosphere; Schlenk technique; Sealed tube 2: N,N-dimethyl-formamide / 21 h / 140 °C / Inert atmosphere; Schlenk technique; Sealed tube View Scheme | |
| Multi-step reaction with 2 steps 1: triethylamine / chloroform-d1 / 60 °C / Inert atmosphere; Schlenk technique; Sealed tube 2: N,N-dimethyl-formamide / 21 h / 140 °C / Inert atmosphere; Schlenk technique; Sealed tube View Scheme | |
| Multi-step reaction with 3 steps 1: chloroform-d1 / 24 h / 40 °C / Inert atmosphere; Schlenk technique; Sealed tube 2: 1,4-dioxane / 0.5 h / 50 °C / Inert atmosphere; Schlenk technique 3: N,N-dimethyl-formamide / 21 h / 140 °C / Inert atmosphere; Schlenk technique; Sealed tube View Scheme | |
| Multi-step reaction with 3 steps 1: chloroform-d1 / 24 h / 40 °C / Inert atmosphere; Schlenk technique; Sealed tube 2: 1,4-dioxane / 0.5 h / 50 °C / Inert atmosphere; Schlenk technique 3: N,N-dimethyl-formamide / 21 h / 140 °C / Inert atmosphere; Schlenk technique; Sealed tube View Scheme | |
| Multi-step reaction with 3 steps 1: chloroform-d1 / 24 h / 40 °C / Inert atmosphere; Schlenk technique; Sealed tube 2: N,N-dimethyl-formamide / 1 h / 100 °C / Inert atmosphere; Schlenk technique; Sealed tube 3: N,N-dimethyl-formamide / 21 h / 140 °C / Inert atmosphere; Schlenk technique; Sealed tube View Scheme |

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1,4-dioxane / 0.5 h / 50 °C / Inert atmosphere; Schlenk technique 2: N,N-dimethyl-formamide / 21 h / 140 °C / Inert atmosphere; Schlenk technique; Sealed tube View Scheme | |
| Multi-step reaction with 2 steps 1: 1,4-dioxane / 0.5 h / 50 °C / Inert atmosphere; Schlenk technique 2: N,N-dimethyl-formamide / 21 h / 140 °C / Inert atmosphere; Schlenk technique; Sealed tube View Scheme | |
| Multi-step reaction with 2 steps 1: N,N-dimethyl-formamide / 1 h / 100 °C / Inert atmosphere; Schlenk technique; Sealed tube 2: N,N-dimethyl-formamide / 21 h / 140 °C / Inert atmosphere; Schlenk technique; Sealed tube View Scheme |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 100℃; for 1h; Inert atmosphere; Schlenk technique; Sealed tube; | A 33 %Chromat. B 38 %Chromat. |

-
-
100-11-8
1-bromomethyl-4-nitro-benzene

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

-
-
1357448-32-8
1,3-bis(2,6-diisopropylphenyl)-2-(4-nitrobenzylidene)-2,3-dihydro-1H-imidazole

| Conditions | Yield |
|---|---|
| Stage #1: 1,3-bis[2,6-diisopropylphenyl]imidazolium chloride With potassium tert-butylate In tetrahydrofuran at 20℃; for 8h; Inert atmosphere; Stage #2: 1-bromomethyl-4-nitro-benzene In tetrahydrofuran at 20℃; for 38h; Inert atmosphere; | 100% |
| Stage #1: 1,3-bis[2,6-diisopropylphenyl]imidazolium chloride With potassium tert-butylate; sodium hydride In tetrahydrofuran at 25℃; for 0.25h; Schlenk technique; Inert atmosphere; Stage #2: 1-bromomethyl-4-nitro-benzene In tetrahydrofuran at 48℃; for 72h; Schlenk technique; Inert atmosphere; | 61% |
-
-
15617-18-2, 39958-10-6, 14220-64-5
bis(benzonitrile)palladium(II) dichloride

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| With KO(tBu) In tetrahydrofuran Ar; Pd compd.(2.5 mmol), ligand (2.5 mmol), and KO(tBu) (2.5 mmol) in THF, stirred at room temp. for 12 h, THF soln. of NaCp (5 mmol) added, mixt. stirred overnight at room temp.; chromd.; | 99% |

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

-
-
1311297-12-7
1,3-bis(2,6-diisopropylphenyl)-1H-imidazole-2(3H)-thione

| Conditions | Yield |
|---|---|
| Stage #1: 1,3-bis[2,6-diisopropylphenyl]imidazolium chloride With sulfur In acetone at 40℃; for 0.25h; Stage #2: With triethylamine In acetone at 60℃; | 99% |
| With potassium carbonate; sulfur In acetone at 60℃; for 16h; Reagent/catalyst; | 99% |
| With potassium tert-butylate; sulfur In tetrahydrofuran at 20℃; | 87% |
-
-
98-98-6
2-Picolinic acid

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| With potassium carbonate In tetrahydrofuran for 12h; Inert atmosphere; Reflux; | 99% |
| With caesium carbonate In 1,4-dioxane at 80℃; for 20h; Schlenk technique; Inert atmosphere; | 65% |


-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| In acetone at 60℃; for 1h; | 99% |
| In neat (no solvent) for 0.0833333h; Solvent; Green chemistry; | 99% |
| In acetone at 60℃; for 1h; | 99% |

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| In acetone at 60℃; for 1h; | 99% |
| In acetone at 60℃; for 1h; | 99% |

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| In neat (no solvent) for 0.0833333h; Green chemistry; | 99% |

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| In neat (no solvent) for 0.0833333h; Green chemistry; | 99% |

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| In neat (no solvent) for 0.0833333h; Green chemistry; | 99% |
-
-
230-27-3
7,8-benzoquinoline

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| With potassium carbonate In tetrahydrofuran at 50℃; for 18h; Inert atmosphere; | 99% |


-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| for 0.0833333h; | 99% |

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| for 0.0833333h; | 99% |

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| for 0.0833333h; | 99% |

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| Milling; | 99% |

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| Milling; | 99% |
-
-
64-19-7
acetic acid

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| With Amberlyst A26 hydroxide In methanol; water at 70℃; | 98.9% |
-
-
626-60-8
3-Chloropyridine

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| With sodium carbonate at 80℃; for 16h; Product distribution / selectivity; | 98% |
| With caesium carbonate at 80℃; for 16h; Reactivity; | 93% |
| With potassium carbonate at 80℃; for 16h; Product distribution / selectivity; | 80% |

-
-
107-14-2
chloroacetonitrile

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

-
-
1225274-70-3
[1,3-bis(2,6-diisopropylphenyl)-2,3-dihydro-1H-imidazol-2-ylidene]acetonitrile

| Conditions | Yield |
|---|---|
| Stage #1: 1,3-bis[2,6-diisopropylphenyl]imidazolium chloride With potassium tert-butylate In tetrahydrofuran at 20℃; for 1.5h; Inert atmosphere; Sonication; Stage #2: chloroacetonitrile In tetrahydrofuran at 20℃; for 72h; Inert atmosphere; | 98% |
| With potassium tert-butylate In tetrahydrofuran-d8 at 20℃; anaerobic conditions; ultrasonification; |
-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| With ammonium hexafluorophosphate In water at 20℃; for 0.166667h; Inert atmosphere; | 98% |
| With potassium hexafluorophosphate In water at 20℃; Inert atmosphere; Glovebox; |

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 60℃; for 5h; | 98% |
| With potassium carbonate In ethanol at 60℃; for 5h; Green chemistry; | 98% |
| Stage #1: bis[chloro(1,2,3-trihapto-allylbenzene)palladium(II)]; 1,3-bis[2,6-diisopropylphenyl]imidazolium chloride In acetone at 60℃; Stage #2: With triethylamine In acetone at 60℃; | 77% |
-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| With N-iodo-succinimide In dichloromethane at 40℃; for 3h; Sealed tube; | 98% |
-
-
108-05-4
vinyl acetate

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

-
-
1134205-18-7
2-(1-acetoxy-1-ethyl)-1,3-bis(2,6-diisopropylphenyl)imidazolium chloride

| Conditions | Yield |
|---|---|
| Stage #1: 1,3-bis[2,6-diisopropylphenyl]imidazolium chloride With potassium tert-butylate In tetrahydrofuran for 0.166667h; Stage #2: vinyl acetate In tetrahydrofuran | 97% |
-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| With dimethylsulfide gold(I) chloride; potassium carbonate In acetone at 60℃; for 1h; Reagent/catalyst; Solvent; Temperature; Time; | 97% |
| Multi-step reaction with 2 steps 1: dichloromethane / 0.25 h 2: potassium carbonate / dichloromethane / 1.5 h View Scheme | |
| Multi-step reaction with 2 steps 1: dimethyl sulfoxide; methanol / Schlenk technique; Inert atmosphere 2: dimethyl sulfoxide; methanol / 4 h / 20 °C / Schlenk technique; Inert atmosphere View Scheme | |
| Multi-step reaction with 2 steps 1: dichloromethane / Darkness 2: dichloromethane View Scheme | |
| Multi-step reaction with 2 steps 1: acetone / 0.17 h / 60 °C 2: sodium acetate / acetone / 0.83 h / 60 °C View Scheme |

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| Milling; | 97% |
| In neat (no solvent, solid phase) for 0.166667h; Milling; |
-
-
64443-05-6
tetrakis(actonitrile)copper(I) hexafluorophosphate

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| With sodium t-butanolate In tetrahydrofuran (Ar); glovebox; mixt. of Cu complex, ligand and NaO-t-Bu in THF was stirred for 6 h; filtered through Celite (THF); filtrate mixed with hexane; filtered; elem. anal.; | 96% |

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| In dichloromethane byproducts: LiCl; under inert gas, imidazolinium-compd. dissolved in CH2Cl2, aluminate-compd. in CH2Cl2 added whilst stirring; filtered through Celite, washed with CH2Cl2, solv. removed, elem. anal.; | 96% |
-
-
39929-21-0
(tetrahydrothiophene)gold(I) chloride

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

-
-
1439915-35-1
1,3-bis(2,6-diisopropylphenyl)-1H-imidazol-3-ium dichloroaurate(I)

| Conditions | Yield |
|---|---|
| In dichloromethane for 0.25h; | 96% |
-
-
12354-84-6, 12354-85-7
bis[dichloro(pentamethylcyclopentadienyl)iridium(III)]

-
-
584-08-7
potassium carbonate

-
-
250285-32-6
1,3-bis[2,6-diisopropylphenyl]imidazolium chloride

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; dichloromethane at 24 - 60℃; for 34h; Inert atmosphere; | 96% |

1,3-Bis(2,6-diisopropylphenyl)imidazolium chloride Chemical Properties
IUPAC Name: 1,3-bis[2,6-Di(propan-2-yl)phenyl]imidazol-1-ium
Following is the structure of 1H-Imidazolium,1,3-bis[2,6-bis(1-methylethyl)phenyl]-, chloride (1:1) (CAS NO.250285-32-6):
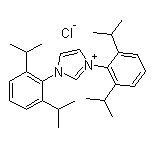
Empirical Formula: C27H37ClN2
Molecular Weight: 425.0491 g/mol
Sensitive: Hygroscopic
Melting point: 278 °C (dec.)(lit.)
Appearance of 1H-Imidazolium,1,3-bis[2,6-bis(1-methylethyl)phenyl]-, chloride (1:1) (CAS NO.250285-32-6): White, off-white or tan powder
Product Categories of 1H-Imidazolium,1,3-bis[2,6-bis(1-methylethyl)phenyl]-, chloride (1:1) (CAS NO.250285-32-6): Imidazolium Compounds; Ligands; N-Heterocyclic Carbene Ligands; Synthetic Organic Chemistry; Catalysis and Inorganic Chemistry; Chemical Synthesis; NHC Compounds
Canonical SMILES: CC(C)C1=C(C(=CC=C1)C(C)C)N2C=C[N+](=C2)C3=C(C=CC=C3C(C)C)C(C)C
InChI: InChI=1S/C27H37N2/c1-18(2)22-11-9-12-23(19(3)4)26(22)28-15-16-29(17-28)27-24(20(5)6)13-10-14-25(27)21(7)8/h9-21H,1-8H3/q+1
InChIKey: VEUHZFXQNDHKGQ-UHFFFAOYSA-N
1,3-Bis(2,6-diisopropylphenyl)imidazolium chloride Toxicity Data With Reference
1H-Imidazolium,1,3-bis[2,6-bis(1-methylethyl)phenyl]-, chloride (1:1) (CAS NO.250285-32-6)hasn't been listed as a carcinogen by ACGIH, IARC, NTP, or CA Prop 65. And the toxicological properties have not been fully investigated.
1,3-Bis(2,6-diisopropylphenyl)imidazolium chloride Safety Profile
Hazard Codes:  Xi
Xi
Risk Statements: 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 26-36
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36:Wear suitable protective clothing.
WGK Germany: 3
1,3-Bis(2,6-diisopropylphenyl)imidazolium chloride Specification
1H-Imidazolium,1,3-bis[2,6-bis(1-methylethyl)phenyl]-, chloride (1:1) , its cas register number is 250285-32-6. It also can be called 1,3-Bis(2,6-diisopropylphenyl)imidazolium chloride .
1H-Imidazolium,1,3-bis[2,6-bis(1-methylethyl)phenyl]-, chloride (1:1) (CAS NO.250285-32-6) is hazardous, so the first aid measures and others should be known. Such as: Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Or in the eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid. While, it's inhaled: Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Then you have the ingesting of the product : Wash mouth out with water, and get medical aid immediately.
1H-Imidazolium,1,3-bis[2,6-bis(1-methylethyl)phenyl]-, chloride (1:1) (CAS NO.250285-32-6) should avoid the condition like incompatible materials. It is not compatible with strong oxidizing agents. And also prevent it to broken down into hazardous decomposition products: hydrogen chloride, nitrogen oxides, carbon monoxide, carbon dioxide. However, its hazardous polymerization has not been reported. In addition, it should be stored in a cool, dry place, or stored in a tightly closed container.
Related Products
- 10,10'-Bis([1,1'-biphenyl]-4-yl)-9,9'-bianthracene
- 10,10'-Dibromo-9,9'-bianthryl
- 10,10-Dimethylanthrone
- 10,10-Oxybisphenoxarsine
- 10-[1,1'-Biphenyl]-4-yl-2-(1-methylethyl)-9-oxo-9H-thioxanthenium hexafluorophosphate
- 10,11-Dehydroimipramine
- 10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine
- 10,11-Dihydro-2-(4-methyl-1-piperazinyl)-11-(2-athiazolyl)-pyridazino(3,4-b)(1,4)benzoxazepine
- 10,11-DIHYDRO-2-(4-METHYL-1-PIPERAZINYL)-11-(3,4-XYLYL)PYRIDAZINO(3,4b)(1,4)-BENZOXAZEPINE MALEATE
- 10,11-Dihydro-5-(3-dimethylamino-2-methylpropyl)-5h-dibenz (b,f)azepine
- 250293-31-3
- 25029-33-8
- 2502-94-5
- 25032-49-9
- 250331-54-5
- 25033-34-5
- 2503-44-8
- 2503-46-0
- 25035-04-5
- 2503-55-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View