-
Name
1,4-DIACETOXYBUTANE
- EINECS
- CAS No. 628-67-1
- Article Data76
- CAS DataBase
- Density 1.039 g/cm3
- Solubility 34.94g/L(26 oC)
- Melting Point 12-15°C
- Formula C8H14O4
- Boiling Point 227.4 °C at 760 mmHg
- Molecular Weight 174.197
- Flash Point 105.2 °C
- Transport Information
- Appearance
- Safety 24/25
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 1,4-Butanediol,diacetate (6CI,7CI,8CI,9CI);1,4-Butylene glycol diacetate;1,4-Diacetoxybutane;Butylene glycol diacetate;NSC 67924;Tetramethyleneacetate;Tetramethylene diacetate;
- PSA 52.60000
- LogP 0.89280
Synthetic route

| Conditions | Yield |
|---|---|
| With dilithium tetra(tert-butyl)zincate In toluene at 0℃; for 1h; Inert atmosphere; | 100% |
| With steapsin lipase In hexane at 55℃; for 24h; Enzymatic reaction; | 99 %Chromat. |
-
-
51326-51-3
4-(tetrahydropyran-2-yloxy)butan-1-ol

-
-
108-24-7
acetic anhydride

-
-
628-67-1
butane-1,4-diol diacetate

| Conditions | Yield |
|---|---|
| With erbium(III) chloride at 50℃; for 0.2h; | 99% |
| cerium triflate In acetonitrile at 20℃; for 4h; | 98% |
| erbium(III) triflate In acetonitrile at 20℃; for 0.5h; |
-
-
108-24-7
acetic anhydride

-
-
87184-99-4
4-(tert-butyldimethylsiloxy)-1-butanol

-
-
628-67-1
butane-1,4-diol diacetate

| Conditions | Yield |
|---|---|
| With erbium(III) chloride at 50℃; for 0.2h; | 99% |
| cerium triflate In acetonitrile at 20℃; for 4h; | 98% |
| erbium(III) triflate In acetonitrile at 20℃; for 0.333333h; |

| Conditions | Yield |
|---|---|
| With sulfuric acid at 20℃; for 20h; | 98% |
| With aminosulfonic acid In acetic acid at 60℃; for 4h; | 97% |
| With zinc(II) chloride at 230℃; |

| Conditions | Yield |
|---|---|
| With benzyl tri-n-butylammonium chloride for 5h; Heating; | 98% |
| With ethanol |

| Conditions | Yield |
|---|---|
| iron(III) sulfate; silica gel for 8h; Heating; | 98% |
| With iodine for 2h; Reflux; chemoselective reaction; | 97% |
| With 8Na(1+)*12C4H10NO(1-)*2HO(1-)*2Nd(3+) In hexane at 60℃; for 16h; Molecular sieve; Inert atmosphere; Schlenk technique; | 92% |
| With aluminum oxide; monoaluminum phosphate at 25℃; | 90% |
| With Novozyme-435 at 20℃; for 24h; Time; Sealed tube; Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| With phosphorus pentoxide; silica gel at 20℃; | 96% |
| samarium(III) trifluoromethanesulfonate at 20℃; for 0.25h; | 96% |
| aluminium dodecatungsten phosphate at 20℃; for 0.133333h; | 94% |

| Conditions | Yield |
|---|---|
| With sulfonated charcoal In benzene for 5h; Heating; | 95% |

| Conditions | Yield |
|---|---|
| With lanthanum(lll) triflate at 180℃; under 15001.5 Torr; for 5h; Inert atmosphere; | 90.4% |
| With sulfuric acid; zinc(II) oxide |

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; for 1h; | 90% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 4h; Acetylation; | 88.9% |

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen; lanthanum(lll) triflate at 180℃; under 15001.5 Torr; for 5h; | 83.2% |
| With palladium 10% on activated carbon; hydrogen; lanthanum(lll) triflate at 180℃; under 19001.3 Torr; for 5h; Reagent/catalyst; Temperature; Pressure; Time; Autoclave; | 68.1% |
-
-
88-14-2
2-furanoic acid

-
-
64-19-7
acetic acid

-
A
-
109-99-9
tetrahydrofuran

-
B
-
628-67-1
butane-1,4-diol diacetate

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen; lanthanum(lll) triflate at 180℃; under 15001.5 Torr; for 5h; Autoclave; | A 6% B 83% |

-
-
75-36-5
acetyl chloride

-
-
82046-44-4
2-ethyl-1,2-oxaalumane

-
A
-
628-67-1
butane-1,4-diol diacetate

-
B
-
141-78-6
ethyl acetate

| Conditions | Yield |
|---|---|
| With oxygen 1.) benzene, 2.) CH2Cl2, 20 deg C, 30 min; 35-40 deg C, 1 h; Yield given. Multistep reaction; | A n/a B 82% |

-
-
110-63-4
Butane-1,4-diol

-
-
141-78-6
ethyl acetate

-
A
-
35435-68-8
4-acetoxy-1-butanol

-
B
-
628-67-1
butane-1,4-diol diacetate

| Conditions | Yield |
|---|---|
| sodium hydrogen sulfate In hexane at 50℃; for 6h; Product distribution; other catalysts, other reaction time; | A 81% B 5% |


| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; for 1h; | 81% |

| Conditions | Yield |
|---|---|
| With polyethylene glycol 400 at 100 - 110℃; for 8h; | 78% |
| at 170℃; |

| Conditions | Yield |
|---|---|
| With indium (III) iodide for 15h; Heating; | 78% |
-
-
88-14-2
2-furanoic acid

-
-
64-19-7
acetic acid

-
A
-
123-86-4
acetic acid butyl ester

-
B
-
628-67-1
butane-1,4-diol diacetate

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen; lanthanum(lll) triflate at 190℃; under 15001.5 Torr; for 5h; Catalytic behavior; Reagent/catalyst; Pressure; Temperature; | A 6.8% B 77.8% |


| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen; lanthanum(lll) triflate at 180℃; under 15001.5 Torr; for 5h; | 76.4% |
| With palladium 10% on activated carbon; hydrogen; lanthanum(lll) triflate at 170℃; under 11400.8 Torr; for 6h; Autoclave; | 60.3% |
-
-
109-99-9
tetrahydrofuran

-
-
108-24-7
acetic anhydride

-
A
-
628-67-1
butane-1,4-diol diacetate

-
B
-
928-88-1
4-(4-acetoxybutoxy)butyl acetate

-
C
-
3216-77-1
1,4-Bis-(4-acetoxy-butyloxy)-butan

| Conditions | Yield |
|---|---|
| With ytterbium(III) triflate at 75 - 80℃; for 1h; | A n/a B 69% C n/a |


| Conditions | Yield |
|---|---|
| With zinc(II) triflate at 150℃; for 24h; | 59% |

| Conditions | Yield |
|---|---|
| With pyridine at 0℃; for 3h; | 26% |
-
-
109-99-9
tetrahydrofuran

-
-
108-24-7
acetic anhydride

-
-
5435-44-9, 22243-66-9
(E)-3-Ureido-but-2-enoic acid ethyl ester

-
-
628-67-1
butane-1,4-diol diacetate

| Conditions | Yield |
|---|---|
| at 230℃; |

-
-
109-99-9
tetrahydrofuran

-
-
108-24-7
acetic anhydride

-
A
-
628-67-1
butane-1,4-diol diacetate

-
B
-
928-88-1
4-(4-acetoxybutoxy)butyl acetate

| Conditions | Yield |
|---|---|
| With diethyl ether; boron trifluoride |


| Conditions | Yield |
|---|---|
| With ethanol; palladium Hydrogenation; |
-
-
110-63-4
Butane-1,4-diol

-
-
60-29-7
diethyl ether

-
-
562-90-3
tetraacetoxysilane

-
-
628-67-1
butane-1,4-diol diacetate

| Conditions | Yield |
|---|---|
| Erhitzen des Reaktionsprodukts; |

-
-
15910-11-9
1,4-diacetoxy 1,3-butadiene

-
-
628-67-1
butane-1,4-diol diacetate

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In methanol |
-
-
628-67-1
butane-1,4-diol diacetate

-
-
35435-68-8
4-acetoxy-1-butanol

| Conditions | Yield |
|---|---|
| With HY-Zeolite In methanol for 5h; Heating; | 97% |
| With sodium hydroxide; phosphate buffer In water for 18h; pH 6.9; | 95% |
| 1,3-disubstituted tetraalkyldistannoxane (X = Y = Cl) In methanol; chloroform for 48h; Ambient temperature; Yield given; |
-
-
35435-68-8
4-acetoxy-1-butanol

-
-
628-67-1
butane-1,4-diol diacetate

-
-
907624-85-5
acetic acid 4-nitryloxybutyl ester

| Conditions | Yield |
|---|---|
| With sulfuric acid; nitric acid In dichloromethane; water at 25℃; | 93% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol for 2h; Reflux; | 60% |
| With hydrogenchloride; methanol; ethanol |

| Conditions | Yield |
|---|---|
| With dilithium tetra(tert-butyl)zincate In toluene at 0℃; for 1h; Inert atmosphere; | 100% |
| With steapsin lipase In hexane at 55℃; for 24h; Enzymatic reaction; | 99 %Chromat. |
-
-
51326-51-3
4-(tetrahydropyran-2-yloxy)butan-1-ol

-
-
108-24-7
acetic anhydride

-
-
628-67-1
butane-1,4-diol diacetate

| Conditions | Yield |
|---|---|
| With erbium(III) chloride at 50℃; for 0.2h; | 99% |
| cerium triflate In acetonitrile at 20℃; for 4h; | 98% |
| erbium(III) triflate In acetonitrile at 20℃; for 0.5h; |
-
-
108-24-7
acetic anhydride

-
-
87184-99-4
4-(tert-butyldimethylsiloxy)-1-butanol

-
-
628-67-1
butane-1,4-diol diacetate

| Conditions | Yield |
|---|---|
| With erbium(III) chloride at 50℃; for 0.2h; | 99% |
| cerium triflate In acetonitrile at 20℃; for 4h; | 98% |
| erbium(III) triflate In acetonitrile at 20℃; for 0.333333h; |

| Conditions | Yield |
|---|---|
| With sulfuric acid at 20℃; for 20h; | 98% |
| With aminosulfonic acid In acetic acid at 60℃; for 4h; | 97% |
| With zinc(II) chloride at 230℃; |

| Conditions | Yield |
|---|---|
| With benzyl tri-n-butylammonium chloride for 5h; Heating; | 98% |
| With ethanol |

| Conditions | Yield |
|---|---|
| iron(III) sulfate; silica gel for 8h; Heating; | 98% |
| With iodine for 2h; Reflux; chemoselective reaction; | 97% |
| With 8Na(1+)*12C4H10NO(1-)*2HO(1-)*2Nd(3+) In hexane at 60℃; for 16h; Molecular sieve; Inert atmosphere; Schlenk technique; | 92% |
| With aluminum oxide; monoaluminum phosphate at 25℃; | 90% |
| With Novozyme-435 at 20℃; for 24h; Time; Sealed tube; Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| With phosphorus pentoxide; silica gel at 20℃; | 96% |
| samarium(III) trifluoromethanesulfonate at 20℃; for 0.25h; | 96% |
| aluminium dodecatungsten phosphate at 20℃; for 0.133333h; | 94% |

| Conditions | Yield |
|---|---|
| With sulfonated charcoal In benzene for 5h; Heating; | 95% |

| Conditions | Yield |
|---|---|
| With lanthanum(lll) triflate at 180℃; under 15001.5 Torr; for 5h; Inert atmosphere; | 90.4% |
| With sulfuric acid; zinc(II) oxide |

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; for 1h; | 90% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 4h; Acetylation; | 88.9% |

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen; lanthanum(lll) triflate at 180℃; under 15001.5 Torr; for 5h; | 83.2% |
| With palladium 10% on activated carbon; hydrogen; lanthanum(lll) triflate at 180℃; under 19001.3 Torr; for 5h; Reagent/catalyst; Temperature; Pressure; Time; Autoclave; | 68.1% |
-
-
88-14-2
2-furanoic acid

-
-
64-19-7
acetic acid

-
A
-
109-99-9
tetrahydrofuran

-
B
-
628-67-1
butane-1,4-diol diacetate

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen; lanthanum(lll) triflate at 180℃; under 15001.5 Torr; for 5h; Autoclave; | A 6% B 83% |

-
-
75-36-5
acetyl chloride

-
-
82046-44-4
2-ethyl-1,2-oxaalumane

-
A
-
628-67-1
butane-1,4-diol diacetate

-
B
-
141-78-6
ethyl acetate

| Conditions | Yield |
|---|---|
| With oxygen 1.) benzene, 2.) CH2Cl2, 20 deg C, 30 min; 35-40 deg C, 1 h; Yield given. Multistep reaction; | A n/a B 82% |

-
-
110-63-4
Butane-1,4-diol

-
-
141-78-6
ethyl acetate

-
A
-
35435-68-8
4-acetoxy-1-butanol

-
B
-
628-67-1
butane-1,4-diol diacetate

| Conditions | Yield |
|---|---|
| sodium hydrogen sulfate In hexane at 50℃; for 6h; Product distribution; other catalysts, other reaction time; | A 81% B 5% |


| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; for 1h; | 81% |

| Conditions | Yield |
|---|---|
| With polyethylene glycol 400 at 100 - 110℃; for 8h; | 78% |
| at 170℃; |

| Conditions | Yield |
|---|---|
| With indium (III) iodide for 15h; Heating; | 78% |
-
-
88-14-2
2-furanoic acid

-
-
64-19-7
acetic acid

-
A
-
123-86-4
acetic acid butyl ester

-
B
-
628-67-1
butane-1,4-diol diacetate

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen; lanthanum(lll) triflate at 190℃; under 15001.5 Torr; for 5h; Catalytic behavior; Reagent/catalyst; Pressure; Temperature; | A 6.8% B 77.8% |


| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen; lanthanum(lll) triflate at 180℃; under 15001.5 Torr; for 5h; | 76.4% |
| With palladium 10% on activated carbon; hydrogen; lanthanum(lll) triflate at 170℃; under 11400.8 Torr; for 6h; Autoclave; | 60.3% |
-
-
109-99-9
tetrahydrofuran

-
-
108-24-7
acetic anhydride

-
A
-
628-67-1
butane-1,4-diol diacetate

-
B
-
928-88-1
4-(4-acetoxybutoxy)butyl acetate

-
C
-
3216-77-1
1,4-Bis-(4-acetoxy-butyloxy)-butan

| Conditions | Yield |
|---|---|
| With ytterbium(III) triflate at 75 - 80℃; for 1h; | A n/a B 69% C n/a |


| Conditions | Yield |
|---|---|
| With zinc(II) triflate at 150℃; for 24h; | 59% |

| Conditions | Yield |
|---|---|
| With pyridine at 0℃; for 3h; | 26% |
-
-
109-99-9
tetrahydrofuran

-
-
108-24-7
acetic anhydride

-
-
5435-44-9, 22243-66-9
(E)-3-Ureido-but-2-enoic acid ethyl ester

-
-
628-67-1
butane-1,4-diol diacetate

| Conditions | Yield |
|---|---|
| at 230℃; |

-
-
109-99-9
tetrahydrofuran

-
-
108-24-7
acetic anhydride

-
A
-
628-67-1
butane-1,4-diol diacetate

-
B
-
928-88-1
4-(4-acetoxybutoxy)butyl acetate

| Conditions | Yield |
|---|---|
| With diethyl ether; boron trifluoride |


| Conditions | Yield |
|---|---|
| With ethanol; palladium Hydrogenation; |
1,4-Butanediol,1,4-diacetate Specification
The 1,4-Butanediol,1,4-diacetate, with CAS registry number 628-67-1, has the systematic name of butane-1,4-diyl diacetate. Besides this, it is also called 4-(Acetyloxy)butyl acetate. And the chemical formula of this chemical is C8H14O4. When use this chemical, please avoid contact with skin and eyes.
Physical properties of 1,4-Butanediol,1,4-diacetate: (1)ACD/LogP: 0.96; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0.96; (4)ACD/LogD (pH 7.4): 0.96; (5)ACD/BCF (pH 5.5): 3.18; (6)ACD/BCF (pH 7.4): 3.18; (7)ACD/KOC (pH 5.5): 79.66; (8)ACD/KOC (pH 7.4): 79.66; (9)#H bond acceptors: 4; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 7; (12)Polar Surface Area: 52.6 Å2; (13)Index of Refraction: 1.422; (14)Molar Refractivity: 42.66 cm3; (15)Molar Volume: 167.5 cm3; (16)Polarizability: 16.91×10-24cm3; (17)Surface Tension: 32.4 dyne/cm; (18)Density: 1.039 g/cm3; (19)Flash Point: 105.2 °C; (20)Enthalpy of Vaporization: 46.4 kJ/mol; (21)Boiling Point: 227.4 °C at 760 mmHg; (22)Vapour Pressure: 0.0777 mmHg at 25°C.
Preparation: this chemical can be prepared by tetrahydrofuran and acetic acid anhydride. This reaction will need reagent HCl and solvent acetic acid.
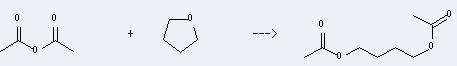
Uses of 1,4-Butanediol,1,4-diacetate: it can be used to produce acetic acid-(4-hydroxy-butyl ester). This reaction will need solvents methanol, CHCl3. The reaction time is 48 hour(s).
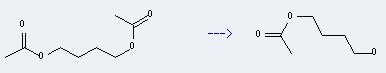
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(OCCCCOC(=O)C)C
(2)InChI: InChI=1/C8H14O4/c1-7(9)11-5-3-4-6-12-8(2)10/h3-6H2,1-2H3
(3)InChIKey: XUKSWKGOQKREON-UHFFFAOYAS
(4)Std. InChI: InChI=1S/C8H14O4/c1-7(9)11-5-3-4-6-12-8(2)10/h3-6H2,1-2H3
(5)Std. InChIKey: XUKSWKGOQKREON-UHFFFAOYSA-N
Related Products
- 10,10'-Bis([1,1'-biphenyl]-4-yl)-9,9'-bianthracene
- 10,10'-Dibromo-9,9'-bianthryl
- 10,10-Dimethylanthrone
- 10,10-Oxybisphenoxarsine
- 10-[1,1'-Biphenyl]-4-yl-2-(1-methylethyl)-9-oxo-9H-thioxanthenium hexafluorophosphate
- 10,11-Dehydroimipramine
- 10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine
- 10,11-Dihydro-2-(4-methyl-1-piperazinyl)-11-(2-athiazolyl)-pyridazino(3,4-b)(1,4)benzoxazepine
- 10,11-DIHYDRO-2-(4-METHYL-1-PIPERAZINYL)-11-(3,4-XYLYL)PYRIDAZINO(3,4b)(1,4)-BENZOXAZEPINE MALEATE
- 10,11-Dihydro-5-(3-dimethylamino-2-methylpropyl)-5h-dibenz (b,f)azepine
- 62867-63-4
- 62868-08-0
- 628-68-2
- 62868-39-7
- 62868-67-1
- 6286-90-4
- 628691-93-0
- 628692-15-9
- 628692-17-1
- 628692-22-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View