-
Name
1-Chloropinacolone
- EINECS 236-920-6
- CAS No. 13547-70-1
- Article Data11
- CAS DataBase
- Density 0.988 g/cm3
- Solubility Not miscible in water.
- Melting Point -13 °C(lit.)
- Formula C6H11ClO
- Boiling Point 171.5 °C at 760 mmHg
- Molecular Weight 134.606
- Flash Point 61.8 °C
- Transport Information UN 2810
- Appearance clear slightly yellow liquid
- Safety 26-36-45-38-36/37/39-28A
- Risk Codes 22-23-36/37/38-26-24
-
Molecular Structure
-
Hazard Symbols
 T,
T, T+,
T+, Xi
Xi
- Synonyms 1-Chloro-3,3-dimethyl-2-butanone;3,3-Dimethyl-2-oxobutyl chloride;Chloromethyl tert-butylketone;tert-Butyl chloromethyl ketone;a-Chloropinacolone;
- PSA 17.07000
- LogP 1.84040
Synthetic route
-
-
75-97-8
3,3-dimethyl-butan-2-one

-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

| Conditions | Yield |
|---|---|
| With chlorine In methanol; (2S)-N-methyl-1-phenylpropan-2-amine hydrate | 92% |
| With p-toluenesulfonyl chloride; lithium diisopropyl amide In tetrahydrofuran -78 deg C to room t., 1 h; | 65% |
| With hydrogenchloride; sodium chlorate at 0℃; for 1h; | 48.8% |
-
-
112449-69-1
pinacoyl (4-methoxyphenyl)tellurium dichloride

-
A
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

-
B
-
35684-37-8
bis(4-methoxyphenyl)ditelluride

| Conditions | Yield |
|---|---|
| at 210℃; under 30 Torr; for 0.0833333h; | A 85% B n/a C n/a |

-
-
17510-46-2
3,3-dimethyl-2-(trimethylsilyl)oxy-1-butene

-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

| Conditions | Yield |
|---|---|
| With copper dichloride In N,N-dimethyl-formamide 1) r.t., 3 h, 2) 50 deg C, 30 min; further reagent: FeCl3 in MeCN; | 65% |
| Multi-step reaction with 2 steps 1: 94 percent / benzene / 10 h / Heating 2: 85 percent / 0.08 h / 210 °C / 30 Torr View Scheme |
-
-
83759-13-1
(E)-1-chloro-3,3-dimethyl-2-methylthiobut-1-ene

-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid at 0℃; for 1h; | 60% |

-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid at 0℃; for 1h; | 60% |

-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid at 0℃; for 1h; | 60% |
-
-
83759-14-2
(Z)-1-chloro-3,3-dimethyl-2-p-tolylthiobut-1-ene

-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid at 0℃; for 1h; | 60% |
-
-
75-97-8
3,3-dimethyl-butan-2-one

-
A
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

-
B
-
22591-21-5
1,1-dichloro-3,3-dimethylbutan-2-one

-
C
-
35039-88-4
1,4-dichloro-3,3-dimethyl-butan-2-one

| Conditions | Yield |
|---|---|
| beim Chlorieren in der Dampfphase; |

-
-
186581-53-3, 908094-01-9
diazomethane

-
-
3282-30-2
pivaloyl chloride

-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

| Conditions | Yield |
|---|---|
| (i), (ii) HCl; Multistep reaction; |
-
-
36402-36-5
(1-dichloromethyl-2,2-dimethyl-propoxy)-trimethyl-silane

-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran; diethyl ether at -110 - -50℃; |
-
-
30263-69-5
1,1-dichloro-3,3-dimethyl-2-butanol

-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

| Conditions | Yield |
|---|---|
| With piperidine; n-butyllithium | |
| at 130 - 140℃; | |
| Multi-step reaction with 2 steps 1: Py / benzene / 18 h / Ambient temperature 2: nBuLi / diethyl ether; tetrahydrofuran / -110 - -50 °C View Scheme |
-
-
14889-24-8
4-chloro-3-methoxy-2,3-dimethyl-butan-2-ol

-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

| Conditions | Yield |
|---|---|
| With sulfuric acid |

-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride |
-
-
76955-39-0
2-tert-Butyl-2-chlorooxirane

-
A
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

-
B
-
4538-80-1
1-fluoro-3,3-dimethyl-2-butanone

| Conditions | Yield |
|---|---|
| With F In diethyl ether at -75℃; | |
| With silver tetrafluoroborate In diethyl ether for 1h; Ambient temperature; Yield given. Yields of byproduct given; |

-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

| Conditions | Yield |
|---|---|
| With sulfuric acid for 0.25h; |
-
-
917-92-0
2,2-dimethyl-3-butyne

-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: CCl4 / Ambient temperature 2: 60 percent / CF3COOH / 1 h / 0 °C View Scheme | |
| Multi-step reaction with 2 steps 1: CHCl3 / Ambient temperature 2: 60 percent / CF3COOH / 1 h / 0 °C View Scheme | |
| Multi-step reaction with 2 steps 1: CCl4 / Ambient temperature 2: 60 percent / CF3COOH / 1 h / 0 °C View Scheme | |
| Multi-step reaction with 2 steps 1: CHCl3 / Ambient temperature 2: 60 percent / CF3COOH / 1 h / 0 °C View Scheme |
-
-
75-97-8
3,3-dimethyl-butan-2-one

-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 78 percent / benzene / 72 h / Heating 2: 85 percent / 0.08 h / 210 °C / 30 Torr View Scheme |
-
-
27843-27-2
2-chloro-3,3-dimethyl-1-butene

-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 60.5 percent / m-chloroperbenzoic acid / CH2Cl2 / 18 h / Ambient temperature 2: AgBF4 / diethyl ether / 1 h / Ambient temperature View Scheme | |
| Multi-step reaction with 2 steps 1: 31 percent / acide m-chloroperoxybenzoique / CH2Cl2 / 15 h / Ambient temperature 2: n-Bu3MePF / diethyl ether / -75 °C View Scheme |
-
-
630-19-3
pivalaldehyde

-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: (i) nBuLi, (ii) /BRN= 506060/ 2: nBuLi, piperidine View Scheme |
-
-
22591-21-5
1,1-dichloro-3,3-dimethylbutan-2-one

-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: LiAlH4, Et2O 2: 130 - 140 °C View Scheme |
-
-
16865-61-5
tert-butyl-2-chloro-acetylene

-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: dimethylformamide; methanol 2: aq. HCl View Scheme |
-
-
75-97-8
3,3-dimethyl-butan-2-one

-
A
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

-
B
-
22591-21-5
1,1-dichloro-3,3-dimethylbutan-2-one

| Conditions | Yield |
|---|---|
| With chlorine; hydrogenchloride at 150℃; Heating / reflux; | |
| With chlorine; hydrogenchloride Heating / reflux; | |
| With chlorine | |
| With 1,3-dichloro-5,5-dimethylhydantoin; silica gel In methanol for 1h; Reflux; |

-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride; formic acid |
-
-
38895-88-4
1-hydroxy-3,3-dimethylbutan-2-one

-
A
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

-
B
-
1025506-09-5
4-tert-butyl-5H-[1,2,3]oxathiazole 2,2-dioxide

| Conditions | Yield |
|---|---|
| With pyridine; formic acid; isocyanate de chlorosulfonyle In acetonitrile at 0 - 10℃; Inert atmosphere; |

-
-
75-97-8
3,3-dimethyl-butan-2-one

-
A
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

-
B
-
22591-21-5
1,1-dichloro-3,3-dimethylbutan-2-one

-
C
-
35039-88-4
1,4-dichloro-3,3-dimethyl-butan-2-one

-
D
-
3205-31-0
2,2,5,6,6-pentamethyl-hept-4-en-3-one

-
E
-
36965-30-7
1,1,1-trichloro-3,3-dimethyl-2-butanone

-
F
-
35039-89-5
1,1,4-trichloro-3,3-dimethyl-butan-2-one

-
G
-
13104-53-5
4-chloro-3,3-dimethylbutane-2-one

-
H
-
35039-91-9
1,4-dichloro-3-chloromethyl-3-methyl-butan-2-one

-
I
-
35039-92-0
1,1,4,4-tetrachloro-3,3-dimethyl-butan-2-one

-
J
-
35039-93-1
1,1,4-trichloro-3-chloromethyl-3-methyl-butan-2-one

| Conditions | Yield |
|---|---|
| With chlorine In tetrachloromethane for 1h; Irradiation; |

-
-
75-97-8
3,3-dimethyl-butan-2-one

-
A
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

-
B
-
13104-53-5
4-chloro-3,3-dimethylbutane-2-one

| Conditions | Yield |
|---|---|
| With sulfuryl dichloride In tetrachloromethane at 20℃; |
-
-
633337-86-7
3'-[4-(hydroxy)-3-methylphenyl]-3'-[4-methylthiophen-2-yl]pentane

-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

-
-
633339-85-2
1-{4-[1-ethyl-1-(4-methyl-thiophen-2-yl)-propyl]-2-methyl-phenoxy}-3,3-dimethyl-butan-2-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone Heating / reflux; | 100% |
| With potassium carbonate In acetone Heating / reflux; | 100% |
-
-
633337-89-0
3'-[4-(hydroxy)-3-methylphenyl]-3'-[5-methoxycarbonyl-4-methylthiophen-2-yl]pentane

-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

-
-
633337-84-5
3'-[4-(2-oxo-3,3-dimethylbutoxy)-3-methylphenyl]-3'-[5-methoxycarbonyl-4-methylthiophen-2-yl]pentane

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone Heating / reflux; | 100% |
| With potassium carbonate In acetone at 20℃; Product distribution / selectivity; Heating / reflux; | 100% |
-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

-
-
76779-98-1
1-azido-3,3-dimethyl-2-butanone

| Conditions | Yield |
|---|---|
| With sodium azide In acetone at 25℃; | 100% |
| With sodium azide In acetone for 36h; Inert atmosphere; | 79% |
| With sodium azide In acetone at 25℃; |
-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

-
-
1445854-08-9
methyl 3-(3-(3-(4-hydroxy-3-methylphenyl)pentan-3-yl)-1H-indol-6-yl)propanoate

-
-
1445854-09-0
methyl 3-(3-(3-(4-(3,3-dimethyl-2-oxobutoxy)-3-methylphenyl)pentan-3-yl)-1H-indol-6-yl)propanoate

| Conditions | Yield |
|---|---|
| With potassium carbonate Inert atmosphere; | 100% |
-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

-
-
630-19-3
pivalaldehyde

-
-
42052-53-9
5-hydroxy-2,2,6,6-tetramethyl-3-heptanone

| Conditions | Yield |
|---|---|
| With samarium diiodide In tetrahydrofuran at -78℃; for 1.41667h; Reformatsky type reaction; Inert atmosphere; | 99% |
-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

-
-
2043-61-0
cyclohexanecarbaldehyde

-
-
65651-67-4
1-cyclohexyl-1-hydroxy-4,4-dimethylpentan-3-one

| Conditions | Yield |
|---|---|
| With samarium diiodide In tetrahydrofuran at -78℃; for 1.41667h; Reformatsky type reaction; Inert atmosphere; | 99% |
-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

-
-
106-49-0
p-toluidine

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In N,N-dimethyl-formamide at 75℃; for 48h; | 99% |
-
-
95903-37-0
7-hydroxy-3-methoxycarbonylmethyl-4-methyl-2H-chromen-2-one

-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

-
-
664365-85-9
methyl [4-methyl-7-(3',3'-dimethyl-2'-oxobutoxy)-2-oxo-2H-benzopyran-3-yl]-acetate

| Conditions | Yield |
|---|---|
| With triethylamine In water at 130℃; for 0.333333h; Microwave irradiation; | 98% |
| With potassium carbonate In acetone at 50 - 56℃; Williamson reaction; | 78% |
-
-
863408-16-6
4-[3-(4-amino-3-methylphenyl)pentan-3-yl]-2-methylphenol

-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

-
-
853404-33-8
1-{4-[3-(4-amino-3-methylphenyl)pentan-3-yl]-2-methylphenoxy}-3,3-dimethylbutan-2-one

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide for 1h; | 97% |
| With sodium hydride In N,N-dimethyl-formamide for 21h; | 97% |

-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In butanone Heating / reflux; | 97% |

-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In butanone Heating / reflux; | 97% |
-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

-
-
59719-19-6
5-(4-chlorophenyl)-2,4-dihydro-3H-pyrazol-3-one

-
-
181067-65-2
3-(4-chlorophenyl)-l-(3, 3-dimethyl-2-oxobutyl)-4, 5-dihydropyrazol-5-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile | 97% |
-
-
252209-94-2
(R)-(-)-1-(2-oxo-5-phenyl-1,3,4,5-tetrahydro-2H-1,5-benzodiazepin-3-yl)-3-[3-(1-methyl-1-tert-butoxycarbonyl)ethylphenyl]urea

-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

| Conditions | Yield |
|---|---|
| Stage #1: (R)-(-)-1-(2-oxo-5-phenyl-1,3,4,5-tetrahydro-2H-1,5-benzodiazepin-3-yl)-3-[3-(1-methyl-1-tert-butoxycarbonyl)ethylphenyl]urea With sodium hydride In N,N-dimethyl-formamide for 1h; Stage #2: 1-chloro-3,3-dimethyl-butan-2-one In N,N-dimethyl-formamide at 20℃; for 1.5h; | 97% |
-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

-
-
38895-88-4
1-hydroxy-3,3-dimethylbutan-2-one

| Conditions | Yield |
|---|---|
| With potassium hydroxide In tetrahydrofuran; water at 40 - 60℃; Reagent/catalyst; | 97% |
| With sodium hydroxide In water at 110℃; for 2h; Reagent/catalyst; Solvent; | 89% |
-
-
500-99-2
3,5-dimethoxyphenol

-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

| Conditions | Yield |
|---|---|
| With potassium hydrogencarbonate; sodium iodide In butanone Heating; | 96% |
-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

-
-
1445853-98-4
methyl (E)-3-(5-(3-(4-hydroxy-3-methylphenyl)pentan-3-yl)-3-methylthiophen-2-yl)acrylate

-
-
1445853-99-5
methyl (E)-3-(5-(3-(4-(3,3-dimethyl-2-oxobutoxy)-3-methylphenyl)pentan-3-yl)-3-methylthiophen-2-yl)acrylate

| Conditions | Yield |
|---|---|
| With potassium carbonate Inert atmosphere; | 95% |
-
-
773837-37-9
sodium cyanide

-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

-
-
59997-51-2
trimethylacetylacetonitrile

| Conditions | Yield |
|---|---|
| With sodium carbonate; sodium iodide In methanol at 60℃; for 3h; | 95% |
-
-
288-88-0
1,2,4-Triazole

-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In ethyl acetate at 50℃; for 5h; | 95% |
-
-
433703-81-2
methyl 2-(7-hydroxy-4,8-dimethyl-2-oxo-2H-chromen-3-yl)acetate

-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

-
-
664365-86-0
methyl [4,8-dimethyl-7-(3',3'-dimethyl-2'-oxobutoxy)-2-oxo-2H-benzopyran-3-yl]-acetate

| Conditions | Yield |
|---|---|
| With triethylamine In water at 130℃; for 0.333333h; Microwave irradiation; | 94% |
| With potassium carbonate In acetone at 50 - 56℃; Williamson reaction; | 81% |
-
-
3069-67-8
2-methyl-1,3,4-oxadiazol-5(4H)-one

-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

| Conditions | Yield |
|---|---|
| Stage #1: 2-methyl-1,3,4-oxadiazol-5(4H)-one With sodium methylate In methanol at 20℃; for 0.25h; Stage #2: 1-chloro-3,3-dimethyl-butan-2-one; tetrabutylammomium bromide In chloroform at 20℃; Heating / reflux; | 94% |

| Conditions | Yield |
|---|---|
| In methanol at 55℃; for 2.5h; Product distribution / selectivity; | 94% |
-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

-
-
540-72-7
sodium thiocyanide

-
-
57518-71-5
3,3-dimethyl-2-oxobutyl thiocyanate

| Conditions | Yield |
|---|---|
| In methanol at 55℃; for 2.5h; | 94% |
-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

-
-
1445854-13-6
methyl 3-(3-(3-(4-hydroxy-3-methylphenyl)pentan-3-yl)-1-methyl-1H-indol-6-yl)propanoate

-
-
1445854-14-7
methyl 3-(3-(3-(4-(3,3-dimethyl-2-oxobutoxy)-3-methylphenyl)pentan-3-yl)-1-methyl-1H-indol-6-yl)propanoate

| Conditions | Yield |
|---|---|
| With potassium carbonate Inert atmosphere; | 94% |
-
-
75-97-8
3,3-dimethyl-butan-2-one

-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

-
-
3205-30-9
5-hydroxy-2,2,5,6,6-pentamethylheptan-3-one

| Conditions | Yield |
|---|---|
| With samarium diiodide In tetrahydrofuran at -78℃; for 2.41667h; Reformatsky type reaction; Inert atmosphere; | 93% |
-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

-
-
45676-04-8
1-tert-butylimidazole

-
-
1266108-71-7
1-(tert-butyl)-3-(3,3-dimethyl-2-oxobutyl)imidazolium chloride

| Conditions | Yield |
|---|---|
| In toluene for 5h; Inert atmosphere; Schlenk technique; Reflux; | 93% |
| In toluene for 24h; Reflux; | 57% |
-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

-
-
1445854-37-4
4-(3-(4-(benzyloxy)phenyl)pentan-3-yl)-2-methylphenol

-
-
1445854-38-5
1-(4-(3-(4-(benzyloxy)phenyl)pentan-3-yl)-2-methylphenoxy)-3,3-dimethylbutan-2-one

| Conditions | Yield |
|---|---|
| With potassium carbonate Inert atmosphere; | 93% |
| With potassium carbonate In acetonitrile for 12h; Reflux; | 93% |
-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

-
-
1445854-18-1
methyl 2-(benzyloxy)-5-(3-(4-hydroxy-3-methylphenyl)-pentan-3-yl)benzoate

-
-
1445854-19-2
methyl 2-(benzyloxy)-5-(3-(4-(3,3-dimethyl-2-oxobutoxy)-3-methylphenyl)pentan-3-yl)benzoate

| Conditions | Yield |
|---|---|
| With potassium carbonate Inert atmosphere; | 93% |
| With potassium carbonate In acetonitrile for 12h; Reflux; | 93% |
-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide; acetone Williamson Ether Synthesis; Heating; | 93% |
-
-
13547-70-1
1-chloro-3,3-dimethyl-butan-2-one

-
-
103-67-3
benzyl-methyl-amine

-
-
877869-93-7
2-(N-benzyl-N-methylamino)-3,3-dimethyl-2-butanone

| Conditions | Yield |
|---|---|
| In 1,2-dimethoxyethane Heating; | 92% |
1-Chloropinacolone Chemical Properties
Molecule structure of 1-Chloropinacolone (CAS NO.13547-70-1):
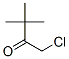
IUPAC Name: 1-Chloropinacolone
Molecular Weight: 134.60394 g/mol
Molecular Formula: C6H11ClO
Density: 0.988 g/cm3
Melting Point: −13 °C(lit.)
Boiling Point: 171.5 °C at 760 mmHg
Flash Point: 61.8 °C
Index of Refraction: 1.423
Molar Refractivity: 34.69 cm3
Molar Volume: 136.1 cm3
Polarizability: 13.75×10-24 cm3
Surface Tension: 26.7 dyne/cm
Enthalpy of Vaporization: 40.79 kJ/mol
Vapour Pressure: 1.39 mmHg at 25 °C
XLogP3-AA: 2
H-Bond Acceptor: 1
Rotatable Bond Count: 2
Tautomer Count: 2
Exact Mass: 134.049843
MonoIsotopic Mass: 134.049843
Topological Polar Surface Area: 17.1
Heavy Atom Count: 8
Complexity: 91.2
Canonical SMILES: CC(C)(C)C(=O)CCl
InChI: InChI=1S/C6H11ClO/c1-6(2,3)5(8)4-7/h4H2,1-3H3
InChIKey: ULSAJQMHTGKPIY-UHFFFAOYSA-N
EINECS: 236-920-6
1-Chloropinacolone Uses
1-Chloropinacolone (CAS NO.13547-70-1) is used as intermediates of medicine, pesticide, etc.
1-Chloropinacolone Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LC50 | inhalation | 4600mg/m3 (4600mg/m3) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 52(12), Pg. 91, 1987. | |
| mouse | LD50 | oral | 355mg/kg (355mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 52(12), Pg. 91, 1987. | |
| rabbit | LD50 | oral | 675mg/kg (675mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 52(12), Pg. 91, 1987. | |
| rat | LD50 | oral | 550mg/kg (550mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 52(12), Pg. 91, 1987. |
1-Chloropinacolone Safety Profile
Hazard Codes:  T,
T,  T+,
T+,  Xi
Xi
Risk Statements: 22-23-36/37/38-26-24
R22:Harmful if swallowed.
R23 :Toxic by inhalation.
R36/37/38:Irritating to eyes, respiratory system and skin.
R26:Very toxic by inhalation.
R34:Causes burns.
Safety Statements: 26-36-45-38-36/37/39-28A
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36:Wear suitable protective clothing.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S38:In case of insufficient ventilation, wear suitable respiratory equipment.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
S28:After contact with skin, wash immediately with plenty of soap-suds.
RIDADR: UN 2810
WGK Germany: 3
RTECS: EL7078000
HazardClass: 6.1
PackingGroup: III
1-Chloropinacolone Specification
1-Chloropinacolone (CAS NO.13547-70-1) is also maned as 1-Chloro-3,3-dimethyl-2-butanone ; 1-Chloropinacolone ; 1-Monochloropinacoline ; Chloromethyl tert-butyl ketone ; Chlorpinakolin ; alpha-Chloropinacolin ; alpha-Chloropinacoline ; tert-Butyl chloromethyl ketone . 1-Chloropinacolone (CAS NO.13547-70-1) clear slightly yellow liquid.
Related Products
- 1-Chloropinacolone
- 135481-57-1
- 135482-70-1
- 13548-38-4
- 1354-84-3
- 135484-51-4
- 135484-74-1
- 135484-76-3
- 135484-78-5
- 135484-83-2
- 13548-68-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View