-
Name
1-Methyl-3-pyrrolidinol
- EINECS 236-189-3
- CAS No. 13220-33-2
- Article Data13
- CAS DataBase
- Density 1.045 g/cm3
- Solubility
- Melting Point
- Formula C5H11NO
- Boiling Point 192.2 °C at 760 mmHg
- Molecular Weight 101.148
- Flash Point 70.6 °C
- Transport Information
- Appearance Colorless or light yellow liquid
- Safety 26-36/37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 1-Methyl-3-pyrrolidinol;N-Methyl-3-hydroxypyrrolidine;N-Methyl-3-pyrrolidinol;NSC 89279;
- PSA 23.47000
- LogP -0.37930
Synthetic route
-
-
19398-47-1
1,4-dibromo-2-butanol

-
-
74-89-5
methylamine

-
A
-
13220-33-2
1-methyl-3-pyrrolidinol

-
B
-
1404453-61-7
1,4-bis(methylamino)butan-2-ol

| Conditions | Yield |
|---|---|
| In water at 100℃; for 16h; Inert atmosphere; | A 98% B 0.9 g |


| Conditions | Yield |
|---|---|
| In water at 10 - 120℃; under 7500.75 Torr; for 10h; Temperature; Pressure; Autoclave; Sealed tube; | 64.8% |

-
-
13220-33-2
1-methyl-3-pyrrolidinol

| Conditions | Yield |
|---|---|
| 56% |

| Conditions | Yield |
|---|---|
| 25% |
-
-
14891-10-2
ethyl-3-oxo-1-pyrrolidinecarboxylate

-
-
13220-33-2
1-methyl-3-pyrrolidinol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride |

| Conditions | Yield |
|---|---|
| With ethanol at 125 - 130℃; |
-
-
554-15-4
1-methyl-2,5-dihydro-pyrrole

-
-
13220-33-2
1-methyl-3-pyrrolidinol

| Conditions | Yield |
|---|---|
| With sodium hydroxide; dimethylsulfide borane complex; dihydrogen peroxide Product distribution; var. hydroborating agents, olefin to hydroborating agent ratios, and times; other N-substituted-3-pyrrolines; |

-
-
13220-33-2
1-methyl-3-pyrrolidinol
-
-
14891-10-2
ethyl-3-oxo-1-pyrrolidinecarboxylate

-
-
60-29-7
diethyl ether

-
-
13220-33-2
1-methyl-3-pyrrolidinol


| Conditions | Yield |
|---|---|
| With sodium tris(acetoxy)borohydride; acetic acid In tetrahydrofuran |
-
-
13220-33-2
1-methyl-3-pyrrolidinol

-
-
1404453-62-8
1-methyl-1-oxypyrrolidin-3-ol

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In ethanol for 21h; Inert atmosphere; | 100% |
-
-
13220-33-2
1-methyl-3-pyrrolidinol

-
-
524-38-9
N-hydroxyphthalimide

-
-
160229-79-8
2-(1-methylpyrrolidin-3-yloxy)isoindoline-1,3-dione

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 0℃; for 6h; Inert atmosphere; | 97% |
-
-
103-82-2
phenylacetic acid

-
-
13220-33-2
1-methyl-3-pyrrolidinol

-
-
434332-38-4
1-methyl-3-pyrrolidinyl phenylacetate

| Conditions | Yield |
|---|---|
| With 4-pyrrolidin-1-ylpyridine; dicyclohexyl-carbodiimide In dichloromethane at 0 - 20℃; | 91% |
-
-
13220-33-2
1-methyl-3-pyrrolidinol

-
-
2309-07-1
Methyl ferulate

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; triphenylphosphine In tetrahydrofuran at 0℃; for 0.5h; | 89% |
-
-
13220-33-2
1-methyl-3-pyrrolidinol

-
-
1476-11-5
Z-1,4-dichlorobutene

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; Inert atmosphere; | 88% |
-
-
13220-33-2
1-methyl-3-pyrrolidinol

-
-
448-19-1
4-fluoro-2-methoxy-1-nitrobenzene

-
-
1001345-84-1
3-(3-methoxy-4-nitro-phenoxy)-1-methyl-pyrrolidine

| Conditions | Yield |
|---|---|
| With potassium hydroxide; tetrabutylammomium bromide In water; toluene at 18 - 60℃; for 18h; | 87% |
| With potassium hydroxide; tetrabutylammomium bromide In water; toluene at 60℃; for 18h; | 87% |
| With sodium hydride In acetonitrile at 80℃; |
-
-
13220-33-2
1-methyl-3-pyrrolidinol

| Conditions | Yield |
|---|---|
| Stage #1: 1-methyl-3-pyrrolidinol With sodium hydride In N,N-dimethyl-formamide at 0℃; for 0.5h; Inert atmosphere; Stage #2: N-(4-fluoro-2-methoxy-5-nitrophenyl)-8-(3-fluorophenyl)quinazolin-2-amine In N,N-dimethyl-formamide at 20℃; for 0.5h; | 86.1% |
-
-
13220-33-2
1-methyl-3-pyrrolidinol

-
-
124-63-0
methanesulfonyl chloride

-
-
91832-73-4
1-methylpyrrolidin-3-yl methanesulfonate

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane at 20℃; for 16h; | 81% |
| With triethylamine In benzene for 0.75h; Ambient temperature; | |
| With triethylamine In dichloromethane at -10 - 20℃; for 2.08333h; | |
| With triethylamine In dichloromethane at 20℃; for 1.5h; Cooling with ice; Inert atmosphere; | |
| With sodium hydroxide In dichloromethane at 0℃; | 124 g |

| Conditions | Yield |
|---|---|
| With azodicarboxylic acid bis(2-methoxyethyl) ester; triphenylphosphine In tetrahydrofuran at 20℃; Cooling with ice; | 78.5% |
-
-
13220-33-2
1-methyl-3-pyrrolidinol

| Conditions | Yield |
|---|---|
| Stage #1: 1-methyl-3-pyrrolidinol With sodium hydride In tetrahydrofuran; mineral oil at 0℃; for 0.25h; Stage #2: (R)-(3-((5-bromopyrimidin-2-yl)amino)pyrrolidin-1-yl)(3-fluoro-4-nitrophenyl)methanone In tetrahydrofuran; mineral oil at 0℃; for 1h; Enzymatic reaction; | 73% |
-
-
109-00-2
3-HYDROXYPYRIDINE

-
-
13220-33-2
1-methyl-3-pyrrolidinol

| Conditions | Yield |
|---|---|
| Stage #1: With triphenylphosphine; diethylazodicarboxylate In tetrahydrofuran at -20℃; for 0.166667h; Stage #2: 1-methyl-3-pyrrolidinol In tetrahydrofuran at -20℃; for 0.166667h; Stage #3: 3-HYDROXYPYRIDINE In tetrahydrofuran at 20℃; Mitsunobu reaction; | 72.9% |
| With triphenylphosphine; diethylazodicarboxylate In tetrahydrofuran Mitsunobu reaction; | |
| With triphenylphosphine; diethylazodicarboxylate In tetrahydrofuran | 260 mg (72.9%) |
-
-
13220-33-2
1-methyl-3-pyrrolidinol

-
-
19833-96-6
2-Cyclopentyl-2-hydroxy-2-phenylacetic acid methyl ester

-
-
13118-11-1
3-(2-cyclopentyl-2-hydroxy-2-phenylacetyloxy)-1-methylpyrrolidine

| Conditions | Yield |
|---|---|
| With sodium In n-heptane for 3h; | 72% |
-
-
13220-33-2
1-methyl-3-pyrrolidinol

-
-
427-49-6
2-cyclopentyl-2-hydroxy-2-phenylacetic acid

-
-
13118-11-1
3-(2-cyclopentyl-2-hydroxy-2-phenylacetyloxy)-1-methylpyrrolidine

| Conditions | Yield |
|---|---|
| Stage #1: 2-cyclopentyl-2-hydroxy-2-phenylacetic acid With 1,1'-carbonyldiimidazole In toluene at 20℃; for 0.5h; Stage #2: 1-methyl-3-pyrrolidinol In toluene at 20℃; | 71% |
| Stage #1: 2-cyclopentyl-2-hydroxy-2-phenylacetic acid With 1,1'-carbonyldiimidazole In N,N-dimethyl-formamide at 18℃; Stage #2: 1-methyl-3-pyrrolidinol In N,N-dimethyl-formamide at 18 - 60℃; for 19h; Product distribution / selectivity; | |
| With 1,1'-carbonyldiimidazole In N,N-dimethyl-formamide at 18 - 60℃; for 19h; Inert atmosphere; | |
| Stage #1: 2-cyclopentyl-2-hydroxy-2-phenylacetic acid With 1,1'-carbonyldiimidazole In N,N-dimethyl-formamide at 20℃; for 0.333333h; Stage #2: 1-methyl-3-pyrrolidinol In N,N-dimethyl-formamide at 60℃; | Ca.580 g |
-
-
64471-47-2
(R)-α-phenyl-α-cyclopentyl-α-hydroxyacetate methyl ester

-
-
13220-33-2
1-methyl-3-pyrrolidinol

-
-
873912-87-9
N-methyl-3-pyrrolidinyl (-)-cyclopentylmandelate

| Conditions | Yield |
|---|---|
| With sodium In n-heptane for 2h; | 70% |
-
-
13220-33-2
1-methyl-3-pyrrolidinol

-
-
1007170-16-2
ethyl 6-(4-chloro-3-hydroxyphenyl)-5-(2,6-dimethoxyphenyl)pyridine-2-carboxylate

-
-
1007170-22-0
Ethyl 6-{4-chloro-3-[(1-methylpyrrolidin-3-yl)oxy]phenyl)-5-(2,6-dimethoxyphenyl)pyridine-2-carboxylate

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triethylamine; triphenylphosphine In tetrahydrofuran at 0 - 20℃; Mitsunobu reaction; Inert atmosphere; | 70% |
-
-
13220-33-2
1-methyl-3-pyrrolidinol

-
-
349-57-5
3-nitro-5-(trifluoromethyl)phenol

-
-
1529769-19-4
1-methyl-3-(3-nitro-5-(trifluoromethyl)phenoxy)pyrrolidine

| Conditions | Yield |
|---|---|
| With triphenylphosphine; diethylazodicarboxylate In tetrahydrofuran at 20℃; for 15h; Cooling with ice; | 67% |
| With triphenylphosphine; diethylazodicarboxylate In tetrahydrofuran at 25℃; for 15h; Cooling with ice; | 67% |
-
-
13220-33-2
1-methyl-3-pyrrolidinol

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
60499-30-1
toluene-4-sulfonic acid 1-methylpyrrolidin-3-yl ester

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane for 5h; | 66% |
| With potassium hydroxide In tetrahydrofuran at 0 - 25℃; for 16h; | 65% |
| With potassium hydroxide In tetrahydrofuran at 0 - 25℃; for 16h; | 44% |
| With dmap; triethylamine In dichloromethane at 20℃; for 16h; | 31% |
| With triethylamine In dichloromethane |
-
-
13220-33-2
1-methyl-3-pyrrolidinol

-
-
295320-15-9
2-(4-fluorophenyl)-1-(4-hydroxybenzyl)-6-(phenylmethoxy)-1,2,3,4-tetrahydroisoquinoline

-
-
998-40-3
tributylphosphine

-
-
10465-81-3
1,1'-(Azodicarbonyl)dipiperidin

-
-
295320-23-9
2-(4-fluorophenyl)-1-[4-(1-methylpyrrolidin-3-yloxy)benzyl]-6-(phenylmethoxy)-1,2,3,4-tetrahydroisoquinoline

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; dichloromethane | 56% |
| In tetrahydrofuran; dichloromethane | 56% |

-
-
13220-33-2
1-methyl-3-pyrrolidinol

-
-
79-44-7
N,N-Dimethylcarbamoyl chloride

| Conditions | Yield |
|---|---|
| With sodium hydride In toluene Ambient temperature; | 52% |
-
-
13220-33-2
1-methyl-3-pyrrolidinol

-
-
32315-10-9
bis(trichloromethyl) carbonate

| Conditions | Yield |
|---|---|
| Stage #1: bis(trichloromethyl) carbonate; (19Z,22Z)-octacosa-19,22-dien-11-ol With pyridine In toluene at 0 - 10℃; for 1.33333h; Stage #2: 1-methyl-3-pyrrolidinol In toluene at 0 - 20℃; | 50% |

-
-
13220-33-2
1-methyl-3-pyrrolidinol

-
-
865886-86-8
5-hydroxy-1-(2-trimethylsilanylethoxymethyl)-1H-indazole-3-carboxylic acid ethyl ester

-
-
869782-61-6
5-(tetrahydro-2H-pyran-4-yloxy)-1-[2-(trimethylsilyl)ethoxy]methyl-1H-indazole-3-carboxylic acid

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran for 16h; Mitsunobu reaction; | 49% |
-
-
13220-33-2
1-methyl-3-pyrrolidinol

-
-
350-46-9
4-Fluoronitrobenzene

-
-
943751-42-6
1-methyl-3-(4-nitrophenoxy)pyrrolidine

| Conditions | Yield |
|---|---|
| Stage #1: 1-methyl-3-pyrrolidinol With potassium tert-butylate In tetrahydrofuran at 20℃; for 1h; Stage #2: 4-Fluoronitrobenzene In tetrahydrofuran for 2h; | 44% |
| With sodium hydride In 1-methyl-pyrrolidin-2-one |
-
-
13220-33-2
1-methyl-3-pyrrolidinol

| Conditions | Yield |
|---|---|
| Stage #1: 2-amino-N-(4-methoxypyridin-3-yl)-5-oxo-4,5-dihydropyrazolo[1,5-a]pyrimidine-3-carboxamide With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; 1,8-diazabicyclo[5.4.0]undec-7-ene In acetonitrile for 0.0833333h; Stage #2: 1-methyl-3-pyrrolidinol With caesium carbonate In acetonitrile at 20℃; for 1h; | 44% |
-
-
13220-33-2
1-methyl-3-pyrrolidinol

-
-
1303954-15-5
2-chloro-8-cyclopentyl-1-propyl-1,7-dihydro-purin-6-one

-
-
1303953-67-4
8-cyclopentyl-2-(1-methylpyrrolidin-3-yl)oxy-1-propyl-7Hpurin-6-one

| Conditions | Yield |
|---|---|
| With sodium hydride In mineral oil for 3h; Inert atmosphere; Reflux; | 41% |
-
-
13220-33-2
1-methyl-3-pyrrolidinol

| Conditions | Yield |
|---|---|
| Stage #1: 2-cyclopentyl-2-hydroxy-2-phenylacetic-1-[1-14C] acid With 1,1'-carbonyldiimidazole In N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: 1-methyl-3-pyrrolidinol In N,N-dimethyl-formamide at 20 - 60℃; | A 41% B n/a |

-
-
13220-33-2
1-methyl-3-pyrrolidinol

-
-
1972-28-7
diethylazodicarboxylate

| Conditions | Yield |
|---|---|
| With hydrogenchloride; triphenylphosphine In methanol; dichloromethane; isopropyl alcohol | 40% |

-
-
13220-33-2
1-methyl-3-pyrrolidinol

-
-
1154427-47-0
3-[2-[2-(3-hydroxy-phenyl)-acetylamino]-2-(2,9,9-trimethyl-3,5-dioxa-4-bora-tricyclo[6.1.1.0(2,6)]dec-4-yl)-ethyl]-benzoic acid tert-butyl ester

-
-
1154427-56-1
3-[2-{2-[3-(1-methyl-pyrrolidin-3-yloxy)-phenyl]-acetylamino}-2-(2,9,9-trimethyl-3,5-dioxa-4-bora-tricyclo[6.1.1.0(2,6)]dec-4-yl)-ethyl]-benzoic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 1-methyl-3-pyrrolidinol; 3-[2-[2-(3-hydroxy-phenyl)-acetylamino]-2-(2,9,9-trimethyl-3,5-dioxa-4-bora-tricyclo[6.1.1.0(2,6)]dec-4-yl)-ethyl]-benzoic acid tert-butyl ester With di-isopropyl azodicarboxylate; triphenylphosphine In dichloromethane Stage #2: With water In dichloromethane | 34% |
1-Methyl-3-pyrrolidinol Chemical Properties
IUPAC Name: 1-methylpyrrolidin-3-ol
Empirical Formula: C5H11NO
Molecular Weight: 101.1469g/mol
EINECS: 236-189-3
Structure of 3-Pyrrolidinol,1-methyl- (CAS NO.13220-33-2):
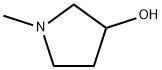
Index of Refraction: 1.496
Molar Refractivity: 28.31 cm3
Molar Volume: 96.7 cm3
Polarizability: 11.22×10-24cm3
Surface Tension: 39 dyne/cm
Density: 1.045 g/cm3
Flash Point: 70.6 °C
Enthalpy of Vaporization: 49.85 kJ/mol
Boiling Point: 192.2 °C at 760 mmHg
Vapour Pressure: 0.132 mmHg at 25°C
Chemical Properties: Clear colorless to pale yellow liquid
Product Categories: Heterocyclic Compounds;Heterocycles;Building Blocks;Heterocyclic Building Blocks;Pyrrolidines
Canonical SMILES: CN1CCC(C1)O
InChI: InChI=1S/C5H11NO/c1-6-3-2-5(7)4-6/h5,7H,2-4H2,1H3
InChIKey: FLVFPAIGVBQGET-UHFFFAOYSA-N
1-Methyl-3-pyrrolidinol Safety Profile
Hazard Codes:  Xi
Xi
Risk Statements: 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 26-36/37/39
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
WGK Germany: 3
1-Methyl-3-pyrrolidinol Specification
3-Pyrrolidinol,1-methyl- , its cas register number is 13220-33-2. It also can be called 1-Methyl-3-pyrrolidinol ; EINECS 236-189-3 ; NSC 89279 . 3-Pyrrolidinol,1-methyl- (CAS NO.13220-33-2) is irritating to eyes, respiratory system and skin. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
Related Products
- 1-Methyl-3-pyrrolidinol
- 132203-70-4
- 132-20-7
- 132207-31-9
- 132208-25-4
- 132210-24-3
- 132213-03-7
- 132213-07-1
- 1322-14-1
- 1322-17-4
- 132-21-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View