-
Name
N-OCTYLPHOSPHONIC ACID
- EINECS 225-218-5
- CAS No. 4724-48-5
- Article Data24
- CAS DataBase
- Density 1.08 g/cm3
- Solubility Slightly soluble in organic solvents and water.
- Melting Point 100-102 °C
- Formula C8H19O3P
- Boiling Point 327.5 °C at 760 mmHg
- Molecular Weight 194.211
- Flash Point 151.8 °C
- Transport Information
- Appearance
- Safety 26-36/37/39
- Risk Codes 36/37/38
-
Molecular Structure
- Hazard Symbols R34:Causes burns.;
- Synonyms Phosphonic acid,octyl- (6CI,7CI,8CI,9CI);Octanephosphonic acid;Octylphosphonic acid;n-Octyl-1-phosphonic acid;n-Octylphosphonic acid;
- PSA 67.34000
- LogP 2.52460
Synthetic route

| Conditions | Yield |
|---|---|
| With hypophosphorous acid; palladium; 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene In water; N,N-dimethyl-formamide at 110℃; for 24h; | 100% |
| With tris-(dibenzylideneacetone)dipalladium(0); hypophosphorous acid; 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene; oxygen In N,N-dimethyl-formamide at 110℃; for 20h; | |
| Multi-step reaction with 2 steps 1: tris-(dibenzylideneacetone)dipalladium(0); 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene / tetrahydrofuran / 9 h / 70 °C 2: sodium hydroxide; potassium permanganate / water / 5 h / 15 °C / Cooling with ice View Scheme |

| Conditions | Yield |
|---|---|
| Stage #1: 1-bromo-octane With aluminum (III) chloride; triethyl phosphite at 20 - 160℃; for 7h; Stage #2: With hydrogen bromide In water for 5h; Reagent/catalyst; Temperature; Reflux; | 95.1% |
| Stage #1: 1-bromo-octane With potassium hydroxide semihydrate; phosphorus; cetyltrimethylammonim bromide In water; toluene at 85 - 90℃; for 6h; Inert atmosphere; Stage #2: With nitric acid In water at 100 - 110℃; for 2h; pH=4; Catalytic behavior; Reagent/catalyst; Temperature; Inert atmosphere; | 70% |
| Multi-step reaction with 2 steps 1: hexane 2: concentrated aqueous HCl View Scheme | |
| Multi-step reaction with 2 steps 2: aq. HCl View Scheme | |
| Multi-step reaction with 2 steps 1: 8 h / 100 °C / Inert atmosphere 2: hydrogen bromide / 2.5 h / 160 °C / Dean-Stark View Scheme |

-
-
4724-48-5
n-octylphosphonic acid

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid In toluene at 100℃; for 24h; Sealed tube; Schlenk technique; Inert atmosphere; | 95% |
| With trifluorormethanesulfonic acid In benzene at 80℃; for 24h; Inert atmosphere; | 72% |

| Conditions | Yield |
|---|---|
| With potassium permanganate; sodium hydroxide In water at 15℃; for 5h; Cooling with ice; | 94% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 24h; Heating; | 86% |
| With hydrogenchloride; sulfuric acid at 35 - 118℃; Temperature; Autoclave; | 82.6% |
| With hydrogen bromide at 160℃; for 2.5h; Dean-Stark; | 70% |
-
-
14576-75-1
diisopropyl octylphosphonate

-
-
4724-48-5
n-octylphosphonic acid

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid In toluene at 120℃; for 50h; Sealed tube; Schlenk technique; Inert atmosphere; | 61% |

| Conditions | Yield |
|---|---|
| With 1,4-dioxane; phosphonic Acid; dibenzoyl peroxide | |
| With 1,4-dioxane; phosphonic Acid; di-tert-butyl peroxide | |
| With phosphoric acid; dibenzoyl peroxide | |
| With 1,4-dioxane; di-tert-butyl peroxide; phosphoric acid |

| Conditions | Yield |
|---|---|
| With hydrogenchloride |

| Conditions | Yield |
|---|---|
| With hydrogenchloride |
-
-
10052-97-8
n-Octyl-phosphinoxid

-
-
4724-48-5
n-octylphosphonic acid

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In methanol |
-
-
111-66-0
oct-1-ene

-
-
110-05-4
di-tert-butyl peroxide

-
-
86119-84-8, 7664-38-2
phosphoric acid

-
A
-
4724-48-5
n-octylphosphonic acid

-
B
-
101440-33-9
2-hexyldecylphosphonic acid

| Conditions | Yield |
|---|---|
| at 90℃; |

-
-
111-66-0
oct-1-ene

-
-
86119-84-8, 7664-38-2
phosphoric acid

-
-
94-36-0
dibenzoyl peroxide

-
A
-
4724-48-5
n-octylphosphonic acid

-
B
-
101440-33-9
2-hexyldecylphosphonic acid

| Conditions | Yield |
|---|---|
| at 90℃; |

-
-
111-66-0
oct-1-ene

-
-
86119-84-8, 7664-38-2
phosphoric acid

-
A
-
4724-48-5
n-octylphosphonic acid

-
B
-
101440-33-9
2-hexyldecylphosphonic acid

| Conditions | Yield |
|---|---|
| im UV-Licht.Irradiation; |

-
-
281199-47-1
2-octyl-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane 2-oxide

-
-
4724-48-5
n-octylphosphonic acid

| Conditions | Yield |
|---|---|
| Stage #1: 2-octyl-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane 2-oxide With trimethylsilyl bromide In dichloromethane at 20℃; for 16h; Stage #2: In methanol; dichloromethane at 20℃; for 2h; Further stages.; |

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); hypophosphorous acid; 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene; oxygen In N,N-dimethyl-formamide at 85℃; Product distribution; Further Variations:; Solvents; Reagents; reactants concentratio; | A 53 % Spectr. B 34 % Spectr. |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 17 h / 33 °C / (γ-irradiation) 2: aq. HCl View Scheme | |
| Multi-step reaction with 3 steps 1: PH3 2: aq. H2O2 / methanol 3: aq. H2O2 / methanol View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: aq. H2O2 / methanol 2: aq. H2O2 / methanol View Scheme |

| Conditions | Yield |
|---|---|
| In water |
-
-
158074-31-8
C14H35O3PSi2

-
-
4724-48-5
n-octylphosphonic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 20 - 50℃; for 22h; |
-
-
17049-49-9
octylmagnesium bromide

-
-
4724-48-5
n-octylphosphonic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: trichlorophosphate / diethyl ether / Inert atmosphere 2: water / diethyl ether / 25.58 h / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1: zinc(II) chloride / diethyl ether / 2.17 h / 0 - 20 °C / Inert atmosphere 2: trichlorophosphate / diethyl ether / 51 h / 0 - 20 °C / Inert atmosphere 3: water / diethyl ether / 25.58 h / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1: cadmium(II) chloride / diethyl ether / 20 °C / Inert atmosphere 2: trichlorophosphate / diethyl ether / 45 h / 0 - 20 °C / Inert atmosphere; Reflux 3: water / diethyl ether / 25.58 h / 20 °C View Scheme |
-
-
64054-30-4
dioctylcadmium

-
-
4724-48-5
n-octylphosphonic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: trichlorophosphate / diethyl ether / 45 h / 0 - 20 °C / Inert atmosphere; Reflux 2: water / diethyl ether / 25.58 h / 20 °C View Scheme |
-
-
14403-26-0
dioctylzinc

-
-
4724-48-5
n-octylphosphonic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: trichlorophosphate / diethyl ether / 51 h / 0 - 20 °C / Inert atmosphere 2: water / diethyl ether / 25.58 h / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| With water In diethyl ether at 20℃; for 25.58h; | 0.49 g |
-
-
4724-48-5
n-octylphosphonic acid

-
-
68254-68-2
zirconyl dichloride hydrate

-
-
807322-41-4
1-hydroxynonyl-1-phosphonic acid

| Conditions | Yield |
|---|---|
| With HF In propan-1-ol; water N2, Zr compd. dissolved in water with HF, a soln. of P compds. (H2O?1-propanol) added dropwise, HF added, refluxed 90-100°C for 5-7 d; ppt. washed (H2O, i-propanol, acetone, diethyl ether), dried (110°C, 24 h); elem. anal.; | 99% |

-
-
4724-48-5
n-octylphosphonic acid

-
-
68254-68-2
zirconyl dichloride hydrate

| Conditions | Yield |
|---|---|
| With hydrofluoric acid In propan-1-ol; water N2, Zr compd. dissolved in water with HF, a soln. of P compd. (H2O/1-propanol) added dropwise, HF added, refluxed 90-100°C for 5-7 d; ppt. washed (H2O, i-propanol, acetone, diethyl ether), dried (110°C, 24 h); elem. anal.; | 95% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In acetonitrile Reflux; | 63% |

-
-
4724-48-5
n-octylphosphonic acid

-
-
64-20-0
tetramethylammonium bromide

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 100℃; | 61% |

-
-
4724-48-5
n-octylphosphonic acid

-
-
72492-60-5
P,P'-(di-n-octyl) dihydrogen pyrophosphonic acid

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In diethyl ether at 20℃; for 0.416667h; | 57% |

| Conditions | Yield |
|---|---|
| With triethylamine at 150℃; for 12h; Autoclave; | 54% |


| Conditions | Yield |
|---|---|
| With triethylamine at 150℃; for 12h; Autoclave; | 53% |

-
-
4724-48-5
n-octylphosphonic acid

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 100℃; | 51% |

-
-
4724-48-5
n-octylphosphonic acid

-
-
74-88-4
methyl iodide

-
-
6172-97-0
octyl-phosphonic acid dimethyl ester

| Conditions | Yield |
|---|---|
| With caesium carbonate In acetonitrile | 35% |
-
-
4724-48-5
n-octylphosphonic acid

-
-
129715-25-9
N-(2-hydroxyethyl)-N,N-dimethyloctane-1-aminium 4-methylbenzenesulphonate

-
-
1616596-39-4
2-[dimetyl(octyl)ammonio]ethyl octylphosphonate

| Conditions | Yield |
|---|---|
| Stage #1: n-octylphosphonic acid With pyridine at 50℃; for 2h; Stage #2: N-(2-hydroxyethyl)-N,N-dimethyloctane-1-aminium 4-methylbenzenesulphonate With pyridine; 2,4,6-triisopropylphenylsulfonyl chloride at 40℃; Stage #3: With water In pyridine at 20℃; for 1h; | 33% |
-
-
4724-48-5
n-octylphosphonic acid

-
-
1616596-38-3
N-(2-hydroxyethyl)-N,N-dimethyl-3,3,4,4,5,5,6,6,7,7,8,8,8-tride-cafluorooctane-1-ammonium tosylate

-
-
1616596-40-7
2-[dimetyl(3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooctyl)ammonio]ethyl octylphosphonate

| Conditions | Yield |
|---|---|
| Stage #1: n-octylphosphonic acid With pyridine at 50℃; for 2h; Stage #2: N-(2-hydroxyethyl)-N,N-dimethyl-3,3,4,4,5,5,6,6,7,7,8,8,8-tride-cafluorooctane-1-ammonium tosylate With pyridine; 2,4,6-triisopropylphenylsulfonyl chloride at 40℃; Stage #3: With water In pyridine at 20℃; for 1h; | 22% |
-
-
16879-02-0
6-chloro-2-hydroxypyridine

-
-
15684-35-2
cobalt(II) tetrafluoroborate hexahydrate

-
-
4724-48-5
n-octylphosphonic acid

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile to soln. Co salt in MeCN 6-chloro-2-hydroxypyridine, RPO3H2 and Et3N were added and stirred for 6 h; soln. was filtered and evapd., residue was extd. with CH2Cl2 and layeredwith hexane; elem. anal.; | 17% |


| Conditions | Yield |
|---|---|
| With phosphorus pentachloride In toluene at 110℃; for 3h; |
-
-
4724-48-5
n-octylphosphonic acid

-
-
76742-18-2
cytidine 5'-monophosphomorpholidate 4-morpholine-N,N'-dicyclohexylcarboxamidine salt

| Conditions | Yield |
|---|---|
| With pyridine at 25 - 35℃; for 80h; |
1-Octylphosphonic acid Specification
The CAS registry number of 1-Octylphosphonic acid is 4724-48-5. Its EINECS registry number is 225-218-5. In addition, the molecular formula is C8H19O3P. The IUPAC name is octylphosphonic acid. What's more, it should be stored in sealed container, and put them in a cool and dry place.
Physical properties about this chemical are: (1)ACD/LogP:1.70; (2)ACD/LogD (pH 5.5):-1.22; (3)ACD/LogD (pH 7.4):-1.85; (4)ACD/BCF (pH 5.5):1; (5)ACD/BCF (pH 7.4):1; (6)ACD/KOC (pH 5.5):1; (7)ACD/KOC (pH 7.4):1; (8)#H bond acceptors:3; (9)#H bond donors:2; (10)#Freely Rotating Bonds:7; (11)Polar Surface Area:45.34 Å2; (12)Index of Refraction:1.458; (13)Molar Refractivity:49.05 cm3; (14)Molar Volume:179.7 cm3; (15)Polarizability:19.44 ×10-24cm3; (16)Surface Tension:41.4 dyne/cm; (17)Density:1.08 g/cm3; (18)Flash Point:151.8 °C; (19)Enthalpy of Vaporization:62.63 kJ/mol; (20)Boiling Point:327.5 °C at 760 mmHg; (21)Vapour Pressure:4.02E-05 mmHg at 25°C.
Preparation of 1-Octylphosphonic acid: it can be prepared by octyl-phosphonic acid diethyl ester. This reaction will need reagent conc. aq. HCl. The reaction time is 24 hours by heating. The yield is about 86%.

Uses of 1-Octylphosphonic acid: it can react with iodomethane to get octyl-phosphonic acid dimethyl ester. This reaction will need reagent Cs2CO3 and solvent acetonitrile. The yield is about 35%.
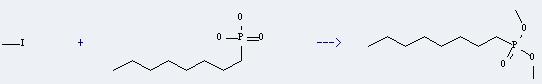
When you are using this chemical, please be cautious about it as the following:
It is irritating to eyes, respiratory system and skin. When you are using it, wear suitable protective clothing, gloves and eye/face protection. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: O=P(O)(O)CCCCCCCC
(2)InChI: InChI=1/C8H19O3P/c1-2-3-4-5-6-7-8-12(9,10)11/h2-8H2,1H3,(H2,9,10,11)
(3)InChIKey: NJGCRMAPOWGWMW-UHFFFAOYAB
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View