-
Name
1-Phenylcyclopentanecarboxylic acid
- EINECS 201-037-7
- CAS No. 77-55-4
- Article Data31
- CAS DataBase
- Density 1.162 g/cm3
- Solubility
- Melting Point 157-161 °C
- Formula C12H14O2
- Boiling Point 338.8 °C at 760 mmHg
- Molecular Weight 190.242
- Flash Point 157.1 °C
- Transport Information
- Appearance Cream to grey-brown powder
- Safety 37/39-26-45-36/37/39-27
- Risk Codes 36/37/38-34
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi, C
C
- Synonyms 1-Phenylcyclopentane-1-carboxylicacid;NSC 19462;
- PSA 37.30000
- LogP 2.58300
Synthetic route

-
-
77-55-4
1-phenyl-1-cyclopentanecarboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: 2-allyl-2-phenylpent-4-enoic acid With tricyclohexylphosphine[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidine][benzylidene]ruthenium(II) dichloride In tetrahydrofuran at 40℃; for 0.5h; Inert atmosphere; Stage #2: With formic acid; sodium hydride In tetrahydrofuran at 80℃; for 24h; Inert atmosphere; | 97% |

-
-
77-55-4
1-phenyl-1-cyclopentanecarboxylic acid

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In water; ethyl acetate at 20℃; for 12h; Green chemistry; regioselective reaction; | 92% |
| With dihydrogen peroxide In ethyl acetate at 20℃; regioselective reaction; | 92% |
-
-
21573-69-3
1-phenyl-1-cyclopentanecarboxaldehyde

-
-
77-55-4
1-phenyl-1-cyclopentanecarboxylic acid

| Conditions | Yield |
|---|---|
| With potassium sulfite; copper(l) chloride; potassium bromide In cyclohexane at 160℃; for 8h; Temperature; | 91% |
| With silver(l) oxide |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol for 96h; Heating; | 68% |
| With sulfuric acid | |
| With potassium hydroxide at 200℃; |
-
-
908520-36-5
1-phenyl-N-(quinolin-8-yl)cyclopentanecarboxamide

-
-
77-55-4
1-phenyl-1-cyclopentanecarboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: 1-phenyl-N-(quinolin-8-yl)cyclopentanecarboxamide With ozone In dichloromethane at -78℃; Inert atmosphere; Stage #2: With lithium hydroxide monohydrate; dihydrogen peroxide In tetrahydrofuran; water at 20℃; for 48h; Inert atmosphere; | 53% |
-
-
110-52-1
1,4-dibromo-butane

-
-
16537-09-0
t-butyl phenylacetate

-
-
77-55-4
1-phenyl-1-cyclopentanecarboxylic acid

| Conditions | Yield |
|---|---|
| (i) aq. NaOH, Cl, DMSO, (ii) aq. H2SO4, AcOH; Multistep reaction; |

| Conditions | Yield |
|---|---|
| With sulfuric acid at 20 - 25℃; for 2h; |

-
-
77-55-4
1-phenyl-1-cyclopentanecarboxylic acid

| Conditions | Yield |
|---|---|
| In water at 37℃; Rate constant; pH 7.4; |

| Conditions | Yield |
|---|---|
| und folgender Hydrolyse des Reaktionsprodukts mit alkoh.KOH; |


| Conditions | Yield |
|---|---|
| With diethyl ether Behandeln des Reaktionsprodukts mit aethanol. Kalilauge; |


| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: NaNH2 2: methanol. KOH / 200 °C View Scheme | |
| Multi-step reaction with 2 steps 1: NaOH 2: aqueous KOH / 130 - 140 °C View Scheme | |
| Multi-step reaction with 2 steps 1: NaNH2 2: KOH; diethylene glycol / 190 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: liq. NH3, NaNH2 2: KOH / bis-(2-hydroxy-ethyl) ether View Scheme | |
| Multi-step reaction with 2 steps 1: NaNH2 2: (acid hydrolysis) View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: toluene / 0.17 h / 20 °C / Inert atmosphere 1.2: 12 h / Inert atmosphere 2.1: dihydrogen peroxide / ethyl acetate; water / 12 h / 20 °C / Green chemistry View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: sodium hydride / mineral oil; methanol; toluene / 12 h / 0 - 20 °C / Inert atmosphere 2.1: toluene / 0.17 h / 20 °C / Inert atmosphere 2.2: 12 h / Inert atmosphere 3.1: dihydrogen peroxide / ethyl acetate; water / 12 h / 20 °C / Green chemistry View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: sodium iodide; sodium hydride / tetrahydrofuran 2.1: sodium hydroxide; water / ethanol / 90 °C 3.1: tricyclohexylphosphine[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidine][benzylidene]ruthenium(II) dichloride / tetrahydrofuran / 0.5 h / 40 °C / Inert atmosphere 3.2: 24 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 2 steps 1.1: sodium hydride / tetrahydrofuran; mineral oil / 0 °C 1.2: 1,4-halobutane / 18.17 h / 0 - 50 °C 2.1: sodium hydroxide / methanol; water / 3 h / Reflux View Scheme |
-
-
242482-24-2
methyl 2-phenyl-2-(prop-2-enyl)pent-4-enoate

-
-
77-55-4
1-phenyl-1-cyclopentanecarboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: sodium hydroxide; water / ethanol / 90 °C 2.1: tricyclohexylphosphine[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidine][benzylidene]ruthenium(II) dichloride / tetrahydrofuran / 0.5 h / 40 °C / Inert atmosphere 2.2: 24 h / 80 °C / Inert atmosphere View Scheme |
-
-
4535-96-0
1-phenyl-1-cyclopentanecarboxylic acid methyl ester

-
-
77-55-4
1-phenyl-1-cyclopentanecarboxylic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol; water for 3h; Reflux; |
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
77-55-4
1-phenyl-1-cyclopentanecarboxylic acid

-
-
4535-96-0
1-phenyl-1-cyclopentanecarboxylic acid methyl ester

| Conditions | Yield |
|---|---|
| In diethyl ether at 20℃; for 24h; | 98% |

| Conditions | Yield |
|---|---|
| With dipotassium hydrogenphosphate; oxygen; palladium diacetate; p-benzoquinone In tetr-butanol at 100℃; under 22801.5 Torr; for 48h; | 98% |
-
-
67-56-1
methanol

-
-
77-55-4
1-phenyl-1-cyclopentanecarboxylic acid

-
-
4535-96-0
1-phenyl-1-cyclopentanecarboxylic acid methyl ester

| Conditions | Yield |
|---|---|
| With thionyl chloride at 20℃; for 17h; Cooling with ice; Inert atmosphere; | 97% |
| With hydrogenchloride for 2h; Heating; | 88% |
| sulfuric acid Heating / reflux; |
-
-
77-55-4
1-phenyl-1-cyclopentanecarboxylic acid

-
-
312727-69-8
phenyl(2,4,6-triisopropylphenyl)iodonium trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In toluene at 0℃; for 1.25h; Reflux; | 94% |
-
-
77-55-4
1-phenyl-1-cyclopentanecarboxylic acid

-
-
59115-90-1
1-hydroxymethyl-1-phenylcyclopentane

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran at 0 - 20℃; for 18h; Reflux; | 93% |
| With borane-THF In tetrahydrofuran for 2h; Ambient temperature; | 75% |
| Multi-step reaction with 2 steps 1: 88 percent / HCl / 2 h / Heating 2: 88 percent / LiAlH4 / tetrahydrofuran / 3 h / Heating View Scheme |

-
-
77-55-4
1-phenyl-1-cyclopentanecarboxylic acid

| Conditions | Yield |
|---|---|
| With (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate; triethylamine In tetrahydrofuran at 20℃; for 2h; | 92% |
-
-
911422-98-5
tert-butyl (1S,4R,5R,7S)-7-amino-5-(3-isopropoxyphenyl)-4-methyl-2-azabicyclo[3.3.1]nonane-2-carboxylate

-
-
77-55-4
1-phenyl-1-cyclopentanecarboxylic acid

-
-
911423-02-4
tert-butyl (1S,4R,5R,7S)-5-(3-isopropoxyphenyl)-4-methyl-7-{[(1-phenyl-1-cyclopentyl)carbonyl]amino}-2-azabicyclo[3.3.1]nonane-2-carboxylate

| Conditions | Yield |
|---|---|
| With (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate; triethylamine In tetrahydrofuran at 20℃; for 4h; | 92% |

-
-
77-55-4
1-phenyl-1-cyclopentanecarboxylic acid

| Conditions | Yield |
|---|---|
| With (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate; triethylamine In tetrahydrofuran at 20℃; for 2h; | 92% |

| Conditions | Yield |
|---|---|
| With (4s,6s)-2,4,5,6-tetra(9H-carbazol-9-yl)isophthalonitrile; caesium carbonate In dimethyl sulfoxide at 40℃; for 1h; Glovebox; Inert atmosphere; Irradiation; | 91% |
-
-
77-55-4
1-phenyl-1-cyclopentanecarboxylic acid

-
-
135569-28-7
1-(4-iodophenyl)cyclopentanecarboxylic acid

| Conditions | Yield |
|---|---|
| With sodium iodate; sulfuric acid; iodine at 20 - 70℃; for 72h; | 89% |
-
-
77-55-4
1-phenyl-1-cyclopentanecarboxylic acid

-
-
52648-77-8
1-(4-nitro-phenyl)-cyclopentanecarboxylic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid; nitric acid at 0 - 20℃; for 3h; | 87% |
| With sulfuric acid; nitric acid In acetic anhydride at 10℃; for 3h; Yield given; |

| Conditions | Yield |
|---|---|
| With C36H24B4N2O3 In toluene at 80℃; for 14h; Molecular sieve; | 86% |
| With oxygen; potassium carbonate; eosin y In toluene at 20℃; Irradiation; Green chemistry; | 86% |
-
-
37622-90-5
4-ethoxycarbonylpyrazole

-
-
77-55-4
1-phenyl-1-cyclopentanecarboxylic acid

| Conditions | Yield |
|---|---|
| With 2,4,6-trimethyl-pyridine; tert-butylammonium hexafluorophosphate(V) In dichloromethane for 2.5h; Molecular sieve; Electrochemical reaction; | 86% |

| Conditions | Yield |
|---|---|
| With 2,6-dimethylpyridine; 4,4'-dichlorodiphenyl disulfide; 10-methyl-9-(2,4,6-trimethylphenyl) acridinium tetrafluoroborate In 1,2-dichloro-ethane at 20℃; for 14h; Irradiation; Inert atmosphere; | 82% |

| Conditions | Yield |
|---|---|
| With nickel(II) bromide dimethoxyethane; 4,4'-Dimethoxy-2,2'-bipyridin; tetrabutylammomium bromide; potassium carbonate In N,N-dimethyl acetamide at 20℃; for 10h; Inert atmosphere; Glovebox; Electrolysis; | 82% |
-
-
77-55-4
1-phenyl-1-cyclopentanecarboxylic acid

-
-
201230-82-2
carbon monoxide

-
-
41058-64-4
1-(2-carboxyphenyl)cyclopentanecarboxylic acid

| Conditions | Yield |
|---|---|
| With dipotassium hydrogenphosphate; sodium acetate; palladium diacetate; silver carbonate In 1,4-dioxane at 150℃; under 760.051 Torr; for 18h; | 80% |
-
-
77-55-4
1-phenyl-1-cyclopentanecarboxylic acid

-
-
423774-22-5
9-(3-amino-3,5-dideoxy-β-D-xylofuranosyl)-N6-cyclopentyladenine

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; diisopropyl-carbodiimide In dichloromethane at 20℃; for 2h; | 79% |

-
-
77-55-4
1-phenyl-1-cyclopentanecarboxylic acid

| Conditions | Yield |
|---|---|
| With (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate; triethylamine In tetrahydrofuran at 20℃; | 78% |

| Conditions | Yield |
|---|---|
| With tetrabutylammonium tetrafluoroborate In methanol; acetonitrile at 20℃; for 6h; Inert atmosphere; Electrochemical reaction; | 77% |
-
-
578-66-5
8-amino quinoline

-
-
77-55-4
1-phenyl-1-cyclopentanecarboxylic acid

-
-
908520-36-5
1-phenyl-N-(quinolin-8-yl)cyclopentanecarboxamide

| Conditions | Yield |
|---|---|
| With thionyl chloride; oxalyl dichloride In dichloromethane; N,N-dimethyl-formamide at 20℃; for 2h; Inert atmosphere; Schlenk technique; | 76% |
-
-
77-55-4
1-phenyl-1-cyclopentanecarboxylic acid

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene; potassium acetate; palladium diacetate; N-acetylglycine In tert-butyl alcohol at 100℃; for 12h; Solvent; Reagent/catalyst; Temperature; Time; Sealed tube; | 69% |
-
-
77-55-4
1-phenyl-1-cyclopentanecarboxylic acid


| Conditions | Yield |
|---|---|
| Stage #1: 1-phenyl-1-cyclopentanecarboxylic acid With N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl acetamide at 20℃; for 0.416667h; Stage #2: 6-(1-methyl-1H-pyrazol-4-yl)-4-(6-(piperazin-1-yl)pyridin-3-yl)pyrazolo[1,5-a]pyridine-3-carbonitrile dihydrochloride With N-ethyl-N,N-diisopropylamine In N,N-dimethyl acetamide | 68% |
-
-
77-55-4
1-phenyl-1-cyclopentanecarboxylic acid

-
-
917-54-4
methyllithium

-
-
4046-09-7
1-(1-phenylcyclopentyl)ethan-1-one

| Conditions | Yield |
|---|---|
| In diethyl ether 1.) 0 deg C; 2.) 2 h, reflux; | 67% |
| In diethyl ether Heating; |
-
-
77-55-4
1-phenyl-1-cyclopentanecarboxylic acid

-
-
59358-70-2
1,1'-Diphenyl-1,1'-bicyclopentyl

| Conditions | Yield |
|---|---|
| With C44H32N4(2+)*2F6P(1-); sodium hydroxide In water; acetonitrile at 20℃; for 18h; Irradiation; | 65% |
| Multi-step reaction with 2 steps 2: sodium; toluene View Scheme |
-
-
77-55-4
1-phenyl-1-cyclopentanecarboxylic acid

-
-
38330-80-2
monomethyl monopotassium malonate

-
-
880352-58-9
3-oxo-3-(1-phenyl-cyclopentyl)-propionic acid methyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 1-phenyl-1-cyclopentanecarboxylic acid With 1,1'-carbonyldiimidazole In tetrahydrofuran at 20℃; for 2h; Stage #2: monomethyl monopotassium malonate With magnesium chloride In tetrahydrofuran at 50℃; | 65% |
-
-
77-55-4
1-phenyl-1-cyclopentanecarboxylic acid

-
-
80735-94-0
1-Phenyl-3-buten-1-ol

| Conditions | Yield |
|---|---|
| With palladium diacetate; silver carbonate In 1,2-dichloro-benzene at 100℃; for 24h; Inert atmosphere; | 65% |
1-Phenylcyclopentanecarboxylic acid Chemical Properties
IUPAC Name: 1-phenylcyclopentane-1-carboxylic acid
The MF of 1-Phenylcyclopentanecarboxylic acid(77-55-4): C12H14O2
The MW of 1-Phenylcyclopentanecarboxylic acid(77-55-4): 190.24
EINECS: 201-037-7
mp: 159-161 °C(lit.)
Density: 1.162 g/cm3
Flash Point: 157.1 °C
Boiling Point: 338.8 °C at 760 mmHg
Index of Refraction: 1.568
Appearance: Cream to gray brown.
Solubility in water: Soluble
Categories: C11 to C12;Carbonyl Compounds;Carboxylic Acids
Synonyms: 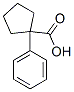 rarechem al bo 1511;phenylcyclopentanecarboxylic acid;akos bc-0226;1-Phenylcyclopentanecarboxylic acid;1-phenylcyclopentane-1-carboxylic acid;1-phenylcyclopentanecarboxylic acid;asischem d50957;1-Phenylcyclopentanecarboxylic acid 99%
rarechem al bo 1511;phenylcyclopentanecarboxylic acid;akos bc-0226;1-Phenylcyclopentanecarboxylic acid;1-phenylcyclopentane-1-carboxylic acid;1-phenylcyclopentanecarboxylic acid;asischem d50957;1-Phenylcyclopentanecarboxylic acid 99%
the structure of 1-Phenylcyclopentanecarboxylic acid(77-55-4):
1-Phenylcyclopentanecarboxylic acid Safety Profile
Hazard Codes:  Xi,
Xi, C
C
Risk Statements: 36/37/38-34
36/37/38: Irritating to eyes, respiratory system and skin
34: Causes burns
Safety Statements: 37/39-26-45-36/37/39-27
37/39: Wear suitable gloves and eye/face protection
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
45: In case of accident or if you feel unwell, seek medical advice immediately (show label where possible)
36/37/39: Wear suitable protective clothing, gloves and eye/face protection
27: Take off immediately all contaminated clothing
WGK Germany: 3
1-Phenylcyclopentanecarboxylic acid Specification
1. First Aid Measures
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water. Get medical aid immediately.
Inhalation:
Get medical aid immediately. Remove from exposure to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen.
Skin:
Get medical aid. Flush skin with plenty of soap and water for at least 15 minutes while removing contaminated clothing and shoes. Remove contaminated clothing and shoes.
Eyes:
Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
2. Handling and Storage
Storage:
Store in a cool, dry place. Keep container closed when not in use.
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View