-
Name
2,3,5-Collidine
- EINECS 211-786-1
- CAS No. 695-98-7
- Article Data17
- CAS DataBase
- Density 0.921 g/cm3
- Solubility slightly soluble in water
- Melting Point -56.98°C (estimate)
- Formula C8H11N
- Boiling Point 187.3 °C at 760 mmHg
- Molecular Weight 121.182
- Flash Point 42.8 °C
- Transport Information
- Appearance clear colourless to very slightly yellow liquid
- Safety 26-36-36/37
- Risk Codes 36/37/38-20/21/22
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn,  Xi,
Xi,  T
T
- Synonyms 2,3,5-Trimethylpyridine;
- PSA 12.89000
- LogP 2.00680
Synthetic route

-
-
695-98-7
2,3,5-trimethyl-pyridine

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran for 1h; Reflux; | 91% |

| Conditions | Yield |
|---|---|
| With Raney nickel at 180℃; for 0.5h; Concentration; Flow reactor; Green chemistry; regioselective reaction; | 90% |
-
-
109371-17-7
2,4-dichloro-3,5,6-trimethylpyridine

-
-
695-98-7
2,3,5-trimethyl-pyridine

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; hydrogen; palladium on activated charcoal In ethanol under 2250.2 Torr; for 3h; further reagent zinc powder, H2SO4; | 83% |
-
-
109371-17-7
2,4-dichloro-3,5,6-trimethylpyridine

-
A
-
695-98-7
2,3,5-trimethyl-pyridine

-
B
-
109371-18-8
4-chloro-2,3,5-trimethylpyridine

-
C
-
121767-77-9
2-chloro-3,5,6-trimethylpyridine

| Conditions | Yield |
|---|---|
| With sulfuric acid; hydrogen; palladium on activated charcoal In ethanol at 20℃; under 1500.1 Torr; | A n/a B 78% C n/a |


| Conditions | Yield |
|---|---|
| With potassium hexafluorophosphate; [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; copper diacetate In tert-Amyl alcohol at 100℃; for 24h; Inert atmosphere; Schlenk technique; regioselective reaction; | 45% |

| Conditions | Yield |
|---|---|
| With diethyl ether; toluene |
-
-
856851-26-8
3-chloromethyl-2,5-dimethyl-pyridine

-
-
695-98-7
2,3,5-trimethyl-pyridine

| Conditions | Yield |
|---|---|
| With methanol; palladium on activated charcoal Hydrogenation; |
-
-
22739-24-8
N-methyl-3,5-dimethylpyridinium iodide

-
A
-
695-98-7
2,3,5-trimethyl-pyridine

-
B
-
3748-84-3
2,3,5,6-tetramethylpyridine

| Conditions | Yield |
|---|---|
| at 300 - 320℃; im Rohr; | |
| at 300 - 320℃; im Rohr; |

| Conditions | Yield |
|---|---|
| With aluminum oxide; ammonia at 330 - 340℃; |

| Conditions | Yield |
|---|---|
| With piperidine hydrochloride In toluene at 200℃; for 2h; autoclave; | 6 % Chromat. |
-
-
7664-41-7
ammonia

-
-
75-07-0
acetaldehyde

-
-
123-38-6
propionaldehyde

-
A
-
583-58-4
3,4-Lutidin

-
B
-
695-98-7
2,3,5-trimethyl-pyridine

-
-
7664-41-7
ammonia

-
-
75-07-0
acetaldehyde

-
-
123-38-6
propionaldehyde

-
A
-
583-58-4
3,4-Lutidin

-
B
-
695-98-7
2,3,5-trimethyl-pyridine

| Conditions | Yield |
|---|---|
| at 330 - 340℃; |

-
-
462-95-3
formaldehyde diethyl acetal

-
-
7664-41-7
ammonia

-
-
123-38-6
propionaldehyde

-
A
-
110-86-1
pyridine

-
B
-
591-22-0
3,5-Lutidine

-
C
-
583-58-4
3,4-Lutidin

-
D
-
695-98-7
2,3,5-trimethyl-pyridine

| Conditions | Yield |
|---|---|
| at 330 - 375℃; aus technischem,acetaldehydhaltigem Propionaldehyd; Produkt 5: β-Picolin; |


| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 74 percent / NaOH, NH2OH*HCl / H2O; ethanol / 1 h / 6 °C 2: 6 percent Chromat. / piperidine*HCl / toluene / 2 h / 200 °C / autoclave View Scheme |
-
-
109371-16-6
4-hydroxy-3,5,6-trimethyl-2(1H)-pyridone

-
-
695-98-7
2,3,5-trimethyl-pyridine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 95 percent / phosphoryl chloride / 4 h / 150 °C 2: 83 percent / hydrogen, conc. NH3 / 10percent Pd/C / ethanol / 3 h / 2250.2 Torr / further reagent zinc powder, H2SO4 View Scheme | |
| Multi-step reaction with 2 steps 1: 95 percent / phosphoryl chloride / 4 h / 150 °C 2: hydrogen, conc. H2SO4 / 10percent Pd/C / ethanol / 20 °C / 1500.1 Torr View Scheme |
-
-
194342-46-6
(2,5-Dimethyl-[3]pyridyl)-methanol

-
-
695-98-7
2,3,5-trimethyl-pyridine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: thionyl chloride 2: palladium/charcoal; methanol / Hydrogenation View Scheme |
-
-
31931-53-0
3-(ethoxycarbonyl)-2,5-dimethylpyridine

-
-
695-98-7
2,3,5-trimethyl-pyridine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: lithium alanate; diethyl ether / und anschliessendes Erwaermen. 2: thionyl chloride 3: palladium/charcoal; methanol / Hydrogenation View Scheme |
-
-
57180-01-5
6-hydroxy-2,5-dimethyl-nicotinic acid ethyl ester

-
-
695-98-7
2,3,5-trimethyl-pyridine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: PCl5 / 160 - 170 °C 2: palladium/charcoal; methanol / Hydrogenation 3: lithium alanate; diethyl ether / und anschliessendes Erwaermen. 4: thionyl chloride 5: palladium/charcoal; methanol / Hydrogenation View Scheme |
-
-
846541-99-9
6-chloro-2,5-dimethyl-nicotinic acid ethyl ester

-
-
695-98-7
2,3,5-trimethyl-pyridine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: palladium/charcoal; methanol / Hydrogenation 2: lithium alanate; diethyl ether / und anschliessendes Erwaermen. 3: thionyl chloride 4: palladium/charcoal; methanol / Hydrogenation View Scheme |
-
-
78-85-3
2-methylpropenal

-
-
78-93-3
butanone

-
A
-
591-22-0
3,5-Lutidine

-
B
-
695-98-7
2,3,5-trimethyl-pyridine

| Conditions | Yield |
|---|---|
| With ammonia; zeolite Gas phase; |

-
-
7664-93-9
sulfuric acid

-
-
109371-17-7
2,4-dichloro-3,5,6-trimethylpyridine

-
-
7440-66-6
zinc

-
-
695-98-7
2,3,5-trimethyl-pyridine

| Conditions | Yield |
|---|---|
| With sodium hydroxide |


-
-
109371-17-7
2,4-dichloro-3,5,6-trimethylpyridine

-
-
695-98-7
2,3,5-trimethyl-pyridine

| Conditions | Yield |
|---|---|
| palladium-carbon In ethanol |

| Conditions | Yield |
|---|---|
| With Ra-Ni for 68h; Reflux; regioselective reaction; |

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; (pentamethylcyclopentadienyl)*Rh(OAc)2; silver(I) 4-methylbenzenesulfonate at 80℃; Reagent/catalyst; | 80 %Spectr. |

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; (pentamethylcyclopentadienyl)*Rh(OAc)2 at 80℃; | A 15 %Spectr. B 15 %Spectr. |


| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: hydroxylamine hydrochloride; sodium carbonate / methanol / 1 h / 65 °C / Inert atmosphere 2: triethylamine / dichloromethane / 0 - 20 °C / Inert atmosphere 3: dipotassium peroxodisulfate; (pentamethylcyclopentadienyl)*Rh(OAc)2 / 80 °C View Scheme |
-
-
20963-51-3, 28051-69-6, 39847-77-3
3-methyl-3-buten-2-one oxime

-
-
695-98-7
2,3,5-trimethyl-pyridine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: triethylamine / dichloromethane / 0 - 20 °C / Inert atmosphere 2: dipotassium peroxodisulfate; (pentamethylcyclopentadienyl)*Rh(OAc)2 / 80 °C View Scheme |

-
-
695-98-7
2,3,5-trimethyl-pyridine

| Conditions | Yield |
|---|---|
| With silver(I) 4-methylbenzenesulfonate at 58℃; Inert atmosphere; |
-
-
695-98-7
2,3,5-trimethyl-pyridine

-
-
638-45-9
1-Iodohexane

-
-
1345623-07-5
1-hexyl-2,3,5-trimethylpyridinium iodide

| Conditions | Yield |
|---|---|
| In toluene at 110℃; for 96h; | 99% |
-
-
695-98-7
2,3,5-trimethyl-pyridine

-
-
77287-34-4, 77287-35-5, 60100-09-6
formamide

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; oxygen; sodium formate; silver nitrate In water at 80℃; for 4h; Schlenk technique; regioselective reaction; | 96% |

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; oxygen; sodium acetate; iron(II) chloride In water at 80℃; for 4h; Green chemistry; | 96% |
-
-
695-98-7
2,3,5-trimethyl-pyridine

-
-
75-09-2
dichloromethane

-
-
7722-84-1
dihydrogen peroxide

-
-
74409-42-0
2,3,5-trimethylpyridine 1-oxide

| Conditions | Yield |
|---|---|
| With sodium hydroxide; acetic acid | 94% |

-
-
695-98-7
2,3,5-trimethyl-pyridine

-
-
87-90-1
trichloroisocyanuric acid

-
-
55-21-0
benzamide

-
-
142885-89-0
2-chloromethyl-3,5-dimethyl-4-nitro-pyridine N-oxide

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water | 93% |

-
-
695-98-7
2,3,5-trimethyl-pyridine

-
-
74409-42-0
2,3,5-trimethylpyridine 1-oxide

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; methyltrioxorhenium(VII) In dichloromethane; water at 20℃; for 3h; | 91% |
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 1 - 20℃; for 13.5h; Product distribution / selectivity; | 88.3% |
| With dihydrogen peroxide In acetic acid at 90 - 95℃; for 9h; | 85% |
-
-
695-98-7
2,3,5-trimethyl-pyridine

-
-
1403873-31-3
1-cyanoethyl trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| In diethyl ether at 20℃; for 48h; Inert atmosphere; | 91% |
-
-
695-98-7
2,3,5-trimethyl-pyridine

-
-
74-88-4
methyl iodide

-
A
-
3748-84-3
2,3,5,6-tetramethylpyridine

-
B
-
1123-96-2
2-ethyl-3,5-dimethylpyridine

| Conditions | Yield |
|---|---|
| Stage #1: 2,3,5-trimethyl-pyridine With n-butyllithium In diethyl ether; hexane for 1.16667h; Inert atmosphere; Stage #2: methyl iodide In diethyl ether; hexane at -78 - 20℃; Inert atmosphere; | A 8% B 85% |

-
-
695-98-7
2,3,5-trimethyl-pyridine

-
-
109-65-9
1-bromo-butane

-
-
1345623-05-3
2-pentyl-3,5-dimethylpyridine

| Conditions | Yield |
|---|---|
| Stage #1: 2,3,5-trimethyl-pyridine With n-butyllithium In diethyl ether; hexane for 1.16667h; Inert atmosphere; Stage #2: 1-bromo-butane In diethyl ether; hexane at -78 - 20℃; Inert atmosphere; | 84% |
-
-
695-98-7
2,3,5-trimethyl-pyridine

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
1104643-33-5
tert-butyl (3,5-dimethylpyridin-2-yl)acetate

| Conditions | Yield |
|---|---|
| Stage #1: 2,3,5-trimethyl-pyridine With lithium diisopropyl amide In tetrahydrofuran; hexane at -78℃; for 0.5h; Stage #2: di-tert-butyl dicarbonate In tetrahydrofuran; hexane at -78 - 20℃; for 2h; | 83% |

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In water; N,N-dimethyl-formamide at 50℃; for 8h; Electrolysis; | 80% |

-
-
695-98-7
2,3,5-trimethyl-pyridine

-
-
321681-28-1
2-bromo-1-(2-bromo-4,5-dimethoxyphenyl)-ethanone

-
-
1268015-48-0
1-[2-(2-bromo-4,5-dimethoxyphenyl)-2-oxoethyl]-2,3,5-trimethylpyridinium bromide

| Conditions | Yield |
|---|---|
| In acetone at 50℃; for 48h; | 79% |
-
-
695-98-7
2,3,5-trimethyl-pyridine

-
-
49851-55-0
2,2'-dibromoacetophenone

-
-
1268015-29-7
1-[2-(2-bromophenyl)-2-oxoethyl]-2,3,5-trimethylpyridinium bromide

| Conditions | Yield |
|---|---|
| In acetone at 50℃; for 48h; | 77% |
-
-
695-98-7
2,3,5-trimethyl-pyridine

-
-
109-72-8, 29786-93-4
n-butyllithium

-
-
74-88-4
methyl iodide

-
A
-
3748-84-3
2,3,5,6-tetramethylpyridine

-
B
-
1123-96-2
2-ethyl-3,5-dimethylpyridine

-
C
-
1345623-05-3
2-pentyl-3,5-dimethylpyridine

| Conditions | Yield |
|---|---|
| Stage #1: 2,3,5-trimethyl-pyridine; n-butyllithium In diethyl ether; hexane for 1.16667h; Inert atmosphere; Stage #2: methyl iodide In diethyl ether; hexane at -78 - 20℃; Inert atmosphere; | A 13% B 13% C 69% |

-
-
695-98-7
2,3,5-trimethyl-pyridine

-
-
109-72-8, 29786-93-4
n-butyllithium

-
-
74-88-4
methyl iodide

-
A
-
1123-96-2
2-ethyl-3,5-dimethylpyridine

-
B
-
1345623-05-3
2-pentyl-3,5-dimethylpyridine

| Conditions | Yield |
|---|---|
| Stage #1: 2,3,5-trimethyl-pyridine; n-butyllithium In diethyl ether; hexane for 1.16667h; Inert atmosphere; Stage #2: methyl iodide In diethyl ether; hexane at -78 - 20℃; Inert atmosphere; | A 27% B 69% |


| Conditions | Yield |
|---|---|
| With oxygen; copper diacetate; trifluoroacetic acid In toluene at 130℃; Schlenk technique; Sealed tube; regioselective reaction; | 63% |
-
-
695-98-7
2,3,5-trimethyl-pyridine

-
-
37052-78-1
2-Mercapto-5-methoxybenzimidazole

-
-
407-25-0
trifluoroacetic anhydride

-
-
73590-58-6
omeprazole

| Conditions | Yield |
|---|---|
| Stage #1: 2,3,5-trimethyl-pyridine With phosphotungstic acid; dihydrogen peroxide In acetonitrile; benzene at 95℃; for 1.5h; Molecular sieve; Stage #2: trifluoroacetic anhydride In chloroform; acetonitrile; benzene for 4.5h; Reflux; Molecular sieve; Stage #3: 2-Mercapto-5-methoxybenzimidazole With sodium hydroxide In ethanol; chloroform; acetonitrile; benzene at 25 - 29℃; for 3h; Temperature; Molecular sieve; | 55.6% |


| Conditions | Yield |
|---|---|
| Stage #1: 2,3,5-trimethyl-pyridine With O-(2,4-dinitrophenyl)hydroxylamine In tetrahydrofuran; water at 40℃; for 12h; Stage #2: p-toluenesulfonyl chloride With sodium hydroxide In tetrahydrofuran; water at 20℃; for 4h; | 26% |
-
-
695-98-7
2,3,5-trimethyl-pyridine

-
A
-
757903-81-4
5,6-dimethylpyridine-3-carboxylic acid

-
B
-
129477-22-1
2,5-dimethyl-nicotinic acid

| Conditions | Yield |
|---|---|
| With permanganate(VII) ion at 20℃; |
-
-
695-98-7
2,3,5-trimethyl-pyridine

| Conditions | Yield |
|---|---|
| With diethyl ether; phenyllithium Behandeln des Reaktionsgemisches mit Kohlensaeure-diaethylester; |
-
-
695-98-7
2,3,5-trimethyl-pyridine

-
-
116668-76-9
pyridine-2,3,5-tricarboxylic acid

| Conditions | Yield |
|---|---|
| With potassium permanganate | |
| With potassium permanganate | |
| Stage #1: 2,3,5-trimethyl-pyridine With potassium permanganate In water at 20 - 70℃; for 14h; Stage #2: With hydrogenchloride In water pH=3; |
2,3,5-Collidine Chemical Properties
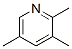
MW: 121.18
bp of 2,3,5-Trimethyl Pyridine (695-98-7): 184 °C(lit.)
density of 2,3,5-Trimethyl Pyridine (695-98-7): 0.931 g/mL at 25 °C(lit.)
refractive index: n20/D 1.508(lit.)
Fp of 2,3,5-Trimethyl Pyridine (695-98-7): 165 °F
Water Solubility: SLIGHTLY SOLUBLE
PHYSICAL STATE: clear to light yellow liquid
2,3,5-Collidine Uses
2,3,5-Collidine Toxicity Data With Reference
Acute Toxicity: LD50 : Not available
2,3,5-Collidine Safety Profile
Risk Statements: 36/37/38-20/21/22 (Irritating to eyes, respiratory system and skin; Harmful by inhalation, in contact with skin and if swallowed)
Safety Statements: 26-36-36/37 (In case of contact with eyes, rinse immediately with plenty of water and seek medical advice; Wear suitable protective clothing; Wear suitable protective clothing and gloves)
WGK Germany: 3
Hazard Note: Toxic
2,3,5-Collidine Standards and Recommendations
LOSS ON DRYING: 0.5% max
MOISTURE: 0.5% max
2,3,5-Collidine Specification
Related Products
- 20,22-DIHYDRODIGITOXIN
- 20,29,30-Trinorlupane,(17alpha)-
- 20-ETHYL-6-β,8-DIHYDROXY-1-α-METHOXY-4-METHYLHETERATISAN-14-ONE
- 20-Ethylprostaglandin F2-alpha
- 20-Isopropylcholanthrene
- 20-METHYLCHOLANTHREN-15-ONE
- 20-METHYLCHOLANTHRENE PICRATE
- 20-METHYLCHOLANTHRENE-TRINITRO-BENZENE
- 20(S)-Ginsenoside C-K
- 2,10-DIFLUOROBENZO(rst)PENTAPHENE
- 695-99-8
- 6960-00-5
- 6960-20-9
- 6960-22-1
- 6960-23-2
- 696-02-6
- 6960-26-5
- 69604-00-8
- 6960-42-5
- 6960-45-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View