-
Name
2,5-Dichloroaniline
- EINECS 202-455-2
- CAS No. 95-82-9
- Article Data72
- CAS DataBase
- Density 1.401 g/cm3
- Solubility water: 0.56 g/L (20 °C)
- Melting Point 48 °C
- Formula C6H5Cl2N
- Boiling Point 250.999 °C at 760 mmHg
- Molecular Weight 162.018
- Flash Point 100.361 °C
- Transport Information UN 3442 6.1/PG 2
- Appearance light brown solid
- Safety 28-36/37-45-60-61-28
- Risk Codes 23/24/25-33-50/53
-
Molecular Structure
-
Hazard Symbols
 T,
T, N
N
- Synonyms Lake Scarlet GG Base;Hiltonil Fast Scarlet 2G Base;Azoene Fast Scarlet 2G Base;Naphtoelan Fast Scarlet GG Base;Fast scarlet 2g base;Naphtoelan fast scarlet GG salt;Amarthol fast scarlet GG salt;Mitsui scarlet GG salt;CI Azoic Diazo Component 3;Symulon scarlet 2g salt;Kayaku Scarlet GG Base;Azogene Fast Scarlet GGC;Spectrolene Scarlet 2G;
- PSA 26.02000
- LogP 3.15680
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogen; zinc dibromide; palladium on activated charcoal In ethyl acetate under 760 Torr; | 100% |
| 99% | |
| With platinum on activated charcoal; hydrogen at 60℃; for 1h; Temperature; Inert atmosphere; Autoclave; Supercritical conditions; | 99.4% |

| Conditions | Yield |
|---|---|
| Stage #1: para-dichlorobenzene With sulfuric acid; nitric acid Stage #2: With platinum on activated charcoal; hydrogen In acetic acid under 3102.97 Torr; | 95% |
| With ammonium hydroxide; bis(acetylacetonate)oxovanadium; tetrabutylammomium bromide; dihydrogen peroxide In acetonitrile at 90℃; for 5h; Reagent/catalyst; Solvent; Time; Molecular sieve; | 57% |
| Multi-step reaction with 2 steps 1: H2SO4; HNO3 2: 88.9 percent / aq. H2SO4 / ethanol / 48 - 50 °C / Electrochemical reaction View Scheme | |
| Multi-step reaction with 2 steps 1: bei der Nitrierung 2: tin; hydrochloric acid View Scheme |
-
-
135145-90-3
2,5-dichlorobenzeneboronic acid

-
-
95-82-9
2,5 dichloroaniline

| Conditions | Yield |
|---|---|
| With copper(I) oxide; ammonium hydroxide; air In methanol at 20℃; for 20h; | 89% |

| Conditions | Yield |
|---|---|
| With sulfuric acid for 3h; Heating; | 82% |
-
-
110-86-1
pyridine

-
-
305-15-7
(2,5-dichlorophenyl)hydrazine

-
A
-
95-82-9
2,5 dichloroaniline

-
B
-
106-46-7
para-dichlorobenzene

-
C
-
4381-30-0
2-(2,5-dichlorophenyl)pyridine

-
D
-
4467-14-5
4-(2,5-Dichloro-phenyl)-pyridine

| Conditions | Yield |
|---|---|
| With KO2 for 10h; Ambient temperature; Yield given. Further byproducts given; | A 6% B 59% C n/a D n/a |

-
-
305-15-7
(2,5-dichlorophenyl)hydrazine

-
A
-
95-82-9
2,5 dichloroaniline

-
B
-
106-46-7
para-dichlorobenzene

-
C
-
4381-30-0
2-(2,5-dichlorophenyl)pyridine

-
D
-
4467-14-5
4-(2,5-Dichloro-phenyl)-pyridine

| Conditions | Yield |
|---|---|
| With KO2 In pyridine for 10h; Ambient temperature; Further byproducts given; | A 6% B 59% C n/a D n/a |


| Conditions | Yield |
|---|---|
| With acetic acid Durch Chlorieren und Destillation des Reaktionsproduktes mit Natronlauge; | |
| With acetic acid Durch Chlorieren; |

| Conditions | Yield |
|---|---|
| With acetic acid Verseifen des Reaktionsproduktes; | |
| With acetic acid und Verseifung des Reaktionsproduktes; |
-
-
67083-41-4
1,4-dichloro-2-nitroso-benzene

-
-
124-41-4
sodium methylate

-
A
-
95-82-9
2,5 dichloroaniline

-
B
-
961-28-4
bis-(2,5-dichloro-phenyl)-diazene-N-oxide


| Conditions | Yield |
|---|---|
| at 230 - 240℃; |
-
-
3032-32-4
3,6-dichloroanthranilic acid

-
A
-
95-82-9
2,5 dichloroaniline

-
B
-
15719-64-9, 15719-76-3, 97762-63-5
methylammonium carbonate

| Conditions | Yield |
|---|---|
| at 230 - 240℃; |

-
A
-
95-82-9
2,5 dichloroaniline

-
B
-
20103-09-7
2,5-dichloro-1,4-phenylenediamine

| Conditions | Yield |
|---|---|
| bei der Reduktion; |

| Conditions | Yield |
|---|---|
| bei der Reduktion; |
-
-
71-23-8
propan-1-ol

-
-
6819-41-6
sodium n-propoxide

-
-
89-61-2
2,5-dichloronitrobenzene

-
-
71-43-2
benzene

-
A
-
95-82-9
2,5 dichloroaniline

-
B
-
99586-92-2
N-(2,5-dichloro-phenyl)-alanine

-
-
141-52-6
sodium ethanolate

-
-
89-61-2
2,5-dichloronitrobenzene

-
-
71-43-2
benzene

-
A
-
95-82-9
2,5 dichloroaniline

-
B
-
961-28-4
bis-(2,5-dichloro-phenyl)-diazene-N-oxide

-
-
89-61-2
2,5-dichloronitrobenzene

-
A
-
95-82-9
2,5 dichloroaniline

-
B
-
43192-05-8
N-(2,5-dichloro-phenyl)-hydroxylamine

| Conditions | Yield |
|---|---|
| With sodium hydrogensulfide; water; calcium chloride |
-
-
64-17-5
ethanol

-
-
53847-61-3
5-methyl-2-phenyl-2H-pyrazole-3,4-dione 4-[(2,5-dichloro-phenyl)-hydrazone]

-
-
64-19-7
acetic acid

-
A
-
95-82-9
2,5 dichloroaniline

| Conditions | Yield |
|---|---|
| durch Oxydation an der Luft in Gegenwart von Eisenchlorid; |


| Conditions | Yield |
|---|---|
| at 245℃; |

| Conditions | Yield |
|---|---|
| at 50 - 100℃; Hydrogenation; |


-
-
7647-01-0
hydrogenchloride

-
-
893-39-0
2,5,2',5'-tetrachlorodiazoaminobenzene

-
A
-
95-82-9
2,5 dichloroaniline

-
B
-
87-87-6
2,3,5,6-tetrachlorobenzene-1,4-diol

| Conditions | Yield |
|---|---|
| at 130℃; |

-
-
7647-01-0
hydrogenchloride

-
-
893-39-0
2,5,2',5'-tetrachlorodiazoaminobenzene

-
A
-
95-82-9
2,5 dichloroaniline

-
B
-
305-15-7
(2,5-dichlorophenyl)hydrazine

-
-
7782-50-5
chlorine

-
-
108-42-9
3-chloro-aniline

-
A
-
95-82-9
2,5 dichloroaniline

-
B
-
634-67-3
2,3,4-trichloroaniline

-
C
-
95-76-1
m,p-dichloroaniline

-
D
-
636-30-6
2,4,5-trichloroaniline

-
-
89-61-2
2,5-dichloronitrobenzene

-
A
-
95-82-9
2,5 dichloroaniline

-
B
-
89-64-5
p-chloro-o-nitrophenol

-
C
-
961-28-4
bis-(2,5-dichloro-phenyl)-diazene-N-oxide


| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: KHCO3; hypochlorous acid 2: glacial acetic acid / und Verseifung des Reaktionsproduktes View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: iodine; chlorine 2: bei der Nitrierung 3: tin; hydrochloric acid View Scheme |

| Conditions | Yield |
|---|---|
| With aniline |

| Conditions | Yield |
|---|---|
| Stage #1: 2,5 dichloroaniline With hydrogenchloride; sodium nitrite In water at 20℃; for 0.166667h; Stage #2: 3-methyl-1-phenylpyrazolin-5-(4H)-one In water at 20℃; for 0.166667h; | 99% |
-
-
19735-89-8
3-methyl-1-phenylpyrazolin-5(4H)-one

-
-
95-82-9
2,5 dichloroaniline

-
-
6407-75-6
pigment yellow 10

| Conditions | Yield |
|---|---|
| Stage #1: 2,5 dichloroaniline With hydrogenchloride; sodium nitrite In water at 20℃; for 0.333333h; Green chemistry; Stage #2: 3-methyl-1-phenylpyrazolin-5(4H)-one In water at 20℃; for 0.166667h; Green chemistry; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: 2,5 dichloroaniline With hydrogenchloride; acetic acid; sodium nitrite In water at 0 - 50℃; Stage #2: tetrafluoroboric acid In water at 5℃; | 98.7% |
-
-
95-82-9
2,5 dichloroaniline

-
-
74220-11-4
2,5-dichloroanilinium hydrogen sulfate

| Conditions | Yield |
|---|---|
| With sulfuric acid In dichloromethane | 98.56% |
-
-
95-82-9
2,5 dichloroaniline

-
-
27761-65-5
2,4-dibromo-3,6-dichloroaniline

| Conditions | Yield |
|---|---|
| With bromine In water at 20℃; for 0.166667h; | 98% |
| With bromine | |
| With N-Bromosuccinimide In tetrahydrofuran at 20℃; for 2h; | |
| With bromine at 20 - 25℃; for 0.0333333h; pH=8.5 - 9; aq. buffer; |
-
-
95-82-9
2,5 dichloroaniline

-
-
79607-22-0, 70271-77-1
6-chloro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid ethyl ester

-
-
1636164-11-8
6-chloro-4-oxo-N'-(2,5-dichlorophenyl)-1,4-dihydroquinoline-3-carboxamide

| Conditions | Yield |
|---|---|
| In diphenylether at 210℃; for 1h; | 98% |
-
-
95-82-9
2,5 dichloroaniline

-
-
81386-60-9
1-hydroxy-9,9-dimethoxy-10-anthrone

| Conditions | Yield |
|---|---|
| In toluene for 18h; Reflux; | 98% |

| Conditions | Yield |
|---|---|
| With fluoro alcohol In water; ethyl acetate; acetonitrile at -20℃; for 0.0833333h; | 97% |
-
-
95-82-9
2,5 dichloroaniline

-
-
140-89-6
potassium ethyl xanthogenate

-
-
5331-91-9
5-chloro-2-mercaptobenzothiazole

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide at 160℃; | 97% |
| In N,N-dimethyl-formamide at 120℃; for 0.2h; microwave irradiation; | 93% |
| for 0.0833333h; Microwave irradiation; | 79% |

-
-
75-77-4
chloro-trimethyl-silane

-
-
95-82-9
2,5 dichloroaniline

-
-
339550-51-5
Mo(VI)[(N-2,5-Cl3C6H2)2Cl2(dimethoxyethane)]

| Conditions | Yield |
|---|---|
| With triethylamine In 1,2-dimethoxyethane byproducts: [(CH3)3Si]2O, NaCl, (C2H5)3NHCl; (Ar), mixed with suspn. of Mo complex, treated with chloromethylsilane, stirred under reflux for 18 h, cooled to room temp.; filtered, washed (DME), evapd.(vac.), cooled to 0°C, elem. anal.,NMR, MAS, XRD; | 97% |

-
-
95-82-9
2,5 dichloroaniline

-
-
99421-72-4
1-phenylethyl 2,2,2-trichloroacetimidate

-
-
1036572-20-9
2,5-dichloro-N-(1-phenylethyl)aniline

| Conditions | Yield |
|---|---|
| With camphor-10-sulfonic acid In dichloromethane at 20℃; for 24h; Reagent/catalyst; Inert atmosphere; | 97% |
-
-
95-82-9
2,5 dichloroaniline

-
-
532-55-8
Benzoyl isothiocyanate

-
-
6281-57-8
N-(2,5-dichlorophenyl)-N'-benzoylthiocarbamide

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 60 - 65℃; for 0.166667h; Microwave irradiation; | 96% |
| In acetone | 79% |
| In acetone | |
| In acetone |
-
-
95-82-9
2,5 dichloroaniline

-
-
66224-70-2
3-(trimethylsilyl)propynoyl chloride

| Conditions | Yield |
|---|---|
| With pyridine In diethyl ether at -50℃; for 1h; Acylation; | 96% |
| In diethyl ether at 25℃; for 1h; |

| Conditions | Yield |
|---|---|
| at 20℃; for 4h; Green chemistry; | 95% |
| at 20℃; for 3.33333h; Irradiation; | 95% |
| at 20℃; for 72h; | 67% |

| Conditions | Yield |
|---|---|
| Stage #1: 2,5 dichloroaniline With hydrogen bromide; sodium nitrite In water at -5 - 0℃; for 0.0833333h; Stage #2: With hydrogen bromide; copper(I) bromide In water at 20 - 50℃; for 0.75h; | 95% |
| Diazotization.Behandlung der Diazoniumsalz-Loesung mit CuBr und HBr; |

| Conditions | Yield |
|---|---|
| Stage #1: 2,5 dichloroaniline With hydrogenchloride; fluoroboric acid; sodium nitrite In water at 25℃; for 0.00555556h; Balz-Schiemann Reaction; Stage #2: In 1,2-dichloro-benzene at 200℃; for 0.0166667h; Balz-Schiemann Reaction; | 95% |
| With hydrogenchloride; tetrafluoroboric acid; sodium nitrite Erhitzen des Reaktionsprodukts; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium nitrite In water at 0 - 20℃; for 3h; | 95% |
| With hydrogenchloride; sodium acetate; sodium nitrite | |
| Stage #1: 2,5 dichloroaniline With hydrogenchloride; sodium nitrite In water at 0 - 5℃; for 0.333333h; Stage #2: 2,5 dichloroaniline In water at 0 - 5℃; for 0.0833333h; |

| Conditions | Yield |
|---|---|
| With calcium(II) chloride dihydrate In neat (no solvent) for 0.166667h; Paal-Knorr Pyrrole Synthesis; Microwave irradiation; Green chemistry; | 95% |
| With Cl(1-)*C5H14NO(1+)*3ZnCl2 In neat (no solvent) at 20℃; for 0.166667h; Paal-Knorr Pyrrole Synthesis; Sealed tube; Sonication; Green chemistry; | 86% |
| With MIL-53(Al) In neat (no solvent) at 80℃; for 0.5h; Paal-Knorr Pyrrole Synthesis; Sonication; | 85% |
-
-
95-82-9
2,5 dichloroaniline

-
A
-
4571-24-8
1,4-dibromo-2,5-dichlorobenzene

-
B
-
73557-61-6
1,2,4-tribromo-3,6-dichlorobenzene

| Conditions | Yield |
|---|---|
| With tert.-butylnitrite; bromine; copper(ll) bromide In acetonitrile at 50℃; for 0.25h; | A 2% B 95% |

| Conditions | Yield |
|---|---|
| With resin D261 In acetonitrile Heating; | 95% |

| Conditions | Yield |
|---|---|
| With Ph3P-Au(phthalimide); C20H38N5(1+)*H(1+)*2BF4(1-) at 50℃; for 4h; Inert atmosphere; | 95% |
| With C20H38N5(1+)*H(1+)*2BF4(1-); C16H8AuN2O4(1-)*C15H29N2(1+) at 50℃; for 4h; Inert atmosphere; | 93% |
| With gallium(III) trichloride at 60℃; for 12h; regioselective reaction; |
-
-
95-82-9
2,5 dichloroaniline

-
-
122835-14-7
5-bromo-2-prop-2-ynyloxy-benzaldehyde

-
-
121-45-9
phosphorous acid trimethyl ester

| Conditions | Yield |
|---|---|
| With triethylamine sulfate In neat (no solvent) at 20℃; for 0.5h; Green chemistry; | 95% |

-
-
95-82-9
2,5 dichloroaniline

| Conditions | Yield |
|---|---|
| With sodium hydroxide; sulfuric acid In 1,2-dichloro-benzene at 35 - 40℃; Product distribution; var. alkaline agents; | 94.1% |
| With potassium hydroxide; sulfuric acid In 1,2-dichloro-benzene at 35 - 40℃; Yield given; |
-
-
50-00-0
formaldehyd

-
-
95-82-9
2,5 dichloroaniline

-
-
461677-71-4
1-benzyl-3-diazo-1,3-dihydro-2H-indol-2-one

-
-
1598427-65-6
1-benzyl-3-((2,5-dichlorophenyl)amino)-3-(hydroxymethyl)indolin-2-one

| Conditions | Yield |
|---|---|
| With dirhodium tetraacetate In water; ethyl acetate at 60℃; for 2h; | 94% |
| With rhodium(II) acetate dimer In ethyl acetate at 60℃; for 2h; | 94% |

-
-
95-82-9
2,5 dichloroaniline

-
-
201230-82-2
carbon monoxide

-
-
610-97-9
o-iodo-methyl-benzoic acid

-
-
1485-35-4
2-(2,5-dichlorophenyl)isoindoline-1,3-dione

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 100℃; under 760.051 Torr; for 1h; Schlenk technique; | 94% |


| Conditions | Yield |
|---|---|
| 93.5% |
-
-
95-82-9
2,5 dichloroaniline

| Conditions | Yield |
|---|---|
| With sulfuric acid In 1,2-dichloro-benzene at 175 - 180℃; 3-5 h; | 93.2% |
-
-
95-82-9
2,5 dichloroaniline

-
-
106-51-4
p-benzoquinone

-
-
79756-69-7
2-(2',5'-dichlorophenyl)-1,4-benzoquinone

| Conditions | Yield |
|---|---|
| Stage #1: 2,5 dichloroaniline With hydrogenchloride; sodium nitrite In water at 0 - 5℃; for 1.5h; Stage #2: p-benzoquinone With sodium acetate In water | 93% |
| Meerwein arylation; | 70% |
2,5-Dichloroaniline Consensus Reports
2,5-Dichloroaniline Specification
The 2,5-Dichloroaniline, with the CAS registry number 95-82-9, is also known as Benzenamine, 2,5-dichloro-. It belongs to the product categories of Intermediates of Dyes and Pigments; Dyes and Pigments; Amines; C2 to C6; Nitrogen Compounds; Alpha Sort; Aromatics Pesticides & Metabolites; Chemical Class; D; DAlphabetic; DIA - DIC. Its EINECS registry number is 202-455-2. This chemical's molecular formula is C6H5Cl2N and molecular weight is 162.02. What's more, its IUPAC name is the same with its product name. It should be stored in a cool, dry and well-ventilated place.
Physical properties about 2,5-Dichloroaniline are: (1)ACD/LogP: 2.715; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.72; (4)ACD/LogD (pH 7.4): 2.72; (5)ACD/BCF (pH 5.5): 68.10; (6)ACD/BCF (pH 7.4): 68.11; (7)ACD/KOC (pH 5.5): 714.10; (8)ACD/KOC (pH 7.4): 714.18; (9)#H bond acceptors: 1; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 26.02 Å2; (13)Index of Refraction: 1.613; (14)Molar Refractivity: 40.279 cm3; (15)Molar Volume: 115.615 cm3; (16)Polarizability: 15.968×10-24 cm3; (17)Surface Tension: 48.33 dyne/cm; (18)Density: 1.401 g/cm3; (19)Flash Point: 100.361 °C; (20)Enthalpy of Vaporization: 48.829 kJ/mol; (21)Boiling Point: 250.999 °C at 760 mmHg; (22)Vapour Pressure: 0.021 mmHg at 25 °C.
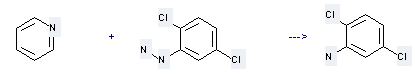
Preparation of 2,5-Dichloroaniline: This chemical can be prepared by pyridine with (2,5-dichloro-phenyl)-hydrazine. This reaction needs reagent KO2 at ambient temperature. The reaction time is 10 hours. The yield is 59 %.
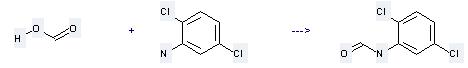
Uses of 2,5-Dichloroaniline: (1) It is used for synthetizing dye intermediates, it is also used in the manufacture of nitrogen fertilizer synergist. (2) It is used to produce other chemicals. For example, it can react with formic acid to get formic acid-(2,5-dichloro-anilide). This reaction temperature is 20 °C. The reaction time is 72 hours. The yield is 67 %.
When you are dealing with this chemical, you should be very careful. This chemical may cause damage to health at low levels. It's very toxic by inhalation, in contact with skin and if swallowed. So you should keep it away from food, drink and animal feeding stuffs. After contacting with skin, you should wash immediately with plenty of abluent which is be specified by the manufacturer. In case of accident or if you feel unwell seek medical advice immediately. In addition, it may present an immediate or delayed danger to one or more components of the environment. This chemical very toxic to aquatic organisms and may cause long-term adverse effects in the aquatic environment. What's more, it may cause danger of cumulative effects. Therefore, this material and its container must be disposed of as hazardous waste. And you should refer to special instructions/safety data sheet before releasing it to the environment.
You can still convert the following datas into molecular structure:
(1) SMILES: Clc1ccc(Cl)c(N)c1
(2) InChI: InChI=1S/C6H5Cl2N/c7-4-1-2-5(8)6(9)3-4/h1-3H,9H2
(3) InChIKey: AVYGCQXNNJPXSS-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LD50 | oral | 3750mg/kg (3750mg/kg) | PERIPHERAL NERVE AND SENSATION: FLACCID PARALYSIS WITHOUT ANESTHESIA (USUALLY NEUROMUSCULAR BLOCKAGE) BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: CYANOSIS | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 33(4), Pg. 8, 1968. |
| mouse | LD50 | intraperitoneal | 400mg/kg (400mg/kg) | Office of Toxic Substances Report. Vol. OTS, | |
| mouse | LD50 | intravenous | 56mg/kg (56mg/kg) | U.S. Army Armament Research & Development Command, Chemical Systems Laboratory, NIOSH Exchange Chemicals. Vol. NX#00202, | |
| mouse | LD50 | oral | 1600mg/kg (1600mg/kg) | Office of Toxic Substances Report. Vol. OTS, | |
| rabbit | LD50 | oral | 3750mg/kg (3750mg/kg) | LUNGS, THORAX, OR RESPIRATION: CYANOSIS BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD PERIPHERAL NERVE AND SENSATION: FLACCID PARALYSIS WITHOUT ANESTHESIA (USUALLY NEUROMUSCULAR BLOCKAGE) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 33(4), Pg. 8, 1968. |
| rat | LD50 | intraperitoneal | 400mg/kg (400mg/kg) | Office of Toxic Substances Report. Vol. OTS, | |
| rat | LD50 | oral | 1600mg/kg (1600mg/kg) | Office of Toxic Substances Report. Vol. OTS, |
Related Products
- 20,22-DIHYDRODIGITOXIN
- 20,29,30-Trinorlupane,(17alpha)-
- 20-ETHYL-6-β,8-DIHYDROXY-1-α-METHOXY-4-METHYLHETERATISAN-14-ONE
- 20-Ethylprostaglandin F2-alpha
- 20-Isopropylcholanthrene
- 20-METHYLCHOLANTHREN-15-ONE
- 20-METHYLCHOLANTHRENE PICRATE
- 20-METHYLCHOLANTHRENE-TRINITRO-BENZENE
- 20(S)-Ginsenoside C-K
- 2,10-DIFLUOROBENZO(rst)PENTAPHENE
- 95-83-0
- 958332-63-3
- 958334-24-2
- 95834-35-8
- 95834-67-6
- 95837-21-1
- 95838-16-7
- 958396-69-5
- 95-84-1
- 958451-71-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View