-
Name
2,5-Diphenyloxazole
- EINECS 202-181-3
- CAS No. 92-71-7
- Article Data135
- CAS DataBase
- Density 1.128 g/cm3
- Solubility NEGLEGIBLE
- Melting Point 72-74 °C(lit.)
- Formula C15H11NO
- Boiling Point 359.998 °C
- Molecular Weight 221.258
- Flash Point 162.302 °C
- Transport Information
- Appearance light beige solid
- Safety 24/25-36/37-26
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 2,5-Diphenyl-1,3-oxazole;DPO (scintillator);NSC 24856;NSC 49168;Tritosol;PPO;PPO(scintillator);
- PSA 26.03000
- LogP 4.00860
Synthetic route
-
-
18735-78-9
2,5-diphenyloxazole-4-carboxylic acid

-
-
92-71-7
2,5-diphenyloxazole

| Conditions | Yield |
|---|---|
| With acetic acid; silver carbonate In dimethyl sulfoxide at 120℃; for 24h; | 100% |

-
-
92-71-7
2,5-diphenyloxazole

| Conditions | Yield |
|---|---|
| With trimethylsilyl trifluoromethanesulfonate In diethyl ether; dichloromethane at -78 - 20℃; for 0.25h; | 100% |
-
-
1006-68-4
5-phenyloxazole

-
-
5123-13-7
5,5-dimethyl-2-phenyl-1,3,2-dioxaborinane

-
-
92-71-7
2,5-diphenyloxazole

| Conditions | Yield |
|---|---|
| With oxygen; copper(l) chloride; sodium t-butanolate In N,N-dimethyl-formamide at 80℃; under 760.051 Torr; for 0.333333h; | 98% |

| Conditions | Yield |
|---|---|
| With sulfuric acid at 30 - 100℃; Temperature; Large scale; | 96.7% |
| With Burgess Reagent In tetrahydrofuran for 0.333333h; Cyclization; Robinson-Gabriel reaction; microwave irradiation; | 93% |
| With sulfuric acid; acetic anhydride at 90℃; | 88% |

| Conditions | Yield |
|---|---|
| With [(tris(3,5-dimethylpyrazolylmethyl)amine)Cu]PF6 at 40℃; for 24h; Reagent/catalyst; Solvent; Inert atmosphere; Schlenk technique; | 95% |

-
-
92-71-7
2,5-diphenyloxazole

| Conditions | Yield |
|---|---|
| In 1,4-dioxane Reflux; | 95% |

| Conditions | Yield |
|---|---|
| With dmap; copper (II)-fluoride; palladium diacetate; 2,2-dimethylpropanoic anhydride; 1,4-di(diphenylphosphino)-butane In 1,4-dioxane at 160℃; for 15h; Schlenk technique; Inert atmosphere; chemoselective reaction; | 95% |

-
-
92-71-7
2,5-diphenyloxazole

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In 1,4-dioxane for 1h; Solvent; Reagent/catalyst; Temperature; Reflux; | 93% |

| Conditions | Yield |
|---|---|
| With triphenylphosphine; silver carbonate; [Pd((diphenylphosphanyl)ferrocene)Cl2]*CH2Cl2 In water at 60℃; for 24h; | 92% |
| With triphenylphosphine; silver carbonate; (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride In dichloromethane; water at 60℃; for 24h; | 92% |
| With caesium carbonate; triphenylphosphine; palladium diacetate In N,N-dimethyl-formamide at 140℃; for 17h; Phenylation; | 79% |

| Conditions | Yield |
|---|---|
| With potassium carbonate; triphenylphosphine; palladium diacetate In N,N-dimethyl-formamide at 140℃; for 17h; Phenylation; | 90% |
-
-
7570-84-5, 51659-21-3, 65309-87-7, 74280-88-9
trans-2-phenyl-3-benzoylaziridine

-
-
92-71-7
2,5-diphenyloxazole

| Conditions | Yield |
|---|---|
| With iodine; dicyclohexyl-carbodiimide In acetonitrile Reagent/catalyst; Solvent; Time; Reflux; | 90% |

| Conditions | Yield |
|---|---|
| With iodine; 4-aminobenzene sulfonic acid In dimethyl sulfoxide at 100℃; for 5h; Reagent/catalyst; Solvent; | 90% |

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); lithium tert-butoxide In 1,4-dioxane at 120℃; for 2h; Inert atmosphere; regioselective reaction; | 89% |
| With copper(l) iodide; potassium carbonate; palladium diacetate In N,N-dimethyl-formamide at 150℃; for 0.25h; microwave irradiation; | 81% |

| Conditions | Yield |
|---|---|
| With potassium phosphate; tris(2,2'-bipyridyl)ruthenium dichloride; Bromotrichloromethane In N,N-dimethyl-formamide at 20℃; Reagent/catalyst; Solvent; Inert atmosphere; Sealed tube; Irradiation; | 88% |
| With iodine; potassium carbonate In N,N-dimethyl-formamide at 80℃; for 4h; Time; Solvent; Reagent/catalyst; | 82% |
| With carbon dioxide; DBN; eosin y In dimethyl sulfoxide at 20℃; for 20h; Reagent/catalyst; Solvent; Schlenk technique; Irradiation; | 76% |
| With tert.-butylhydroperoxide; iodine; caesium carbonate In tetrahydrofuran; water at 60℃; for 12h; Reagent/catalyst; Solvent; Temperature; Green chemistry; | 74% |

| Conditions | Yield |
|---|---|
| With copper(I) bromide In chlorobenzene at 80℃; for 17h; | 88% |
| With oxygen; copper(l) chloride In toluene at 80℃; for 8h; Reagent/catalyst; Solvent; | 82% |

-
-
92-71-7
2,5-diphenyloxazole

| Conditions | Yield |
|---|---|
| With fac-tris(2-phenylpyridinato-N,C2')iridium(III) In 1,4-dioxane at 20℃; for 3h; Catalytic behavior; Solvent; Inert atmosphere; Irradiation; | 88% |
-
-
72997-93-4
1-benzyl-2-benzoyl-3-phenylaziridine

-
-
92-71-7
2,5-diphenyloxazole

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In 1,4-dioxane for 0.5h; Reagent/catalyst; Reflux; | 87% |

-
-
92-71-7
2,5-diphenyloxazole

| Conditions | Yield |
|---|---|
| In toluene at 160℃; for 2h; | 86% |

| Conditions | Yield |
|---|---|
| With [2,2]bipyridinyl; copper (II)-fluoride; nickel(II) bromide 2-methoxyethyl ether complex; cesium fluoride In N,N-dimethyl acetamide at 150℃; Inert atmosphere; | 86% |

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene; ammonium acetate at 20℃; for 5h; Reagent/catalyst; Temperature; | 86% |

-
-
92-71-7
2,5-diphenyloxazole

| Conditions | Yield |
|---|---|
| In cyclohexane at 90℃; for 24h; | 84% |
-
-
78007-47-3
trans-N-styryl-benzamide

-
-
92-71-7
2,5-diphenyloxazole

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; 3-pyridinecarboxylic acid ethyl ester; tetrabutylammomium bromide; copper(ll) bromide In acetonitrile at 20℃; for 24h; Reagent/catalyst; Solvent; Inert atmosphere; | 84% |
| With trimethylsilyl trifluoromethanesulfonate; bis-[(trifluoroacetoxy)iodo]benzene In diethyl ether; dichloromethane at -78 - 0℃; Solvent; Reagent/catalyst; Temperature; | 75% |
| With 1-methyl-1H-imidazole; copper dichloride In 1,4-dioxane at 140℃; for 20h; Sealed tube; | 74 %Spectr. |

| Conditions | Yield |
|---|---|
| With iodine In dimethyl sulfoxide at 100℃; | 84% |

| Conditions | Yield |
|---|---|
| Stage #1: Benzaldoxime With trifluorormethanesulfonic acid; silica gel In toluene at 50℃; for 0.5h; Stage #2: phenylacetylene In toluene at 70℃; for 3.5h; Stage #3: With copper(II) choride dihydrate; lithium chloride hydrate; oxygen; 2,3-dicyano-5,6-dichloro-p-benzoquinone In 1,4-dioxane; propan-1-ol; toluene at 60℃; under 9750.98 Torr; for 4h; Reagent/catalyst; | 84% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; sodium carbonate; triphenylphosphine In N,N-dimethyl-formamide at 160℃; for 2h; Inert atmosphere; | 83% |
| With lithium tert-butoxide In diethyl ether at 30℃; for 24h; Inert atmosphere; Sealed tube; Irradiation; | 76% |
| With copper(l) iodide; dimethylaminoacetic acid; lithium tert-butoxide In diethyl ether at 25 - 27℃; for 16h; Inert atmosphere; Sealed tube; Irradiation; | 74% |

| Conditions | Yield |
|---|---|
| With pyridine; oxygen; potassium carbonate; copper(I) bromide In toluene at 20 - 80℃; under 760.051 Torr; for 16.1667h; Reagent/catalyst; Solvent; Schlenk technique; | 82% |

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; iodine In dimethyl sulfoxide at 80℃; | 81% |

| Conditions | Yield |
|---|---|
| With copper diacetate; palladium diacetate; triphenylphosphine In 1,4-dioxane; dimethyl sulfoxide at 120℃; for 24h; Inert atmosphere; | 81% |

| Conditions | Yield |
|---|---|
| With PdCl2(C3H3N2(CH3))(C3H2N2(C6H3(C3H7)2)2); lithium tert-butoxide In toluene at 130℃; for 18h; Inert atmosphere; | 81% |

| Conditions | Yield |
|---|---|
| With silver carbonate In 1,4-dioxane at 50℃; for 12h; | 78% |
| With pyridine; oxygen; potassium carbonate; lithium bromide; copper(ll) bromide In toluene at 110℃; for 11h; | 74% |
| With tert.-butylhydroperoxide; iodine In dimethyl sulfoxide at 80℃; |
-
-
92-71-7
2,5-diphenyloxazole

| Conditions | Yield |
|---|---|
| With [bis(2-methylallyl)cycloocta-1,5-diene]ruthenium(II); (R,R)-2,2″-bis[(S)-1-diphenylphosphinoethyl]-1,1″-biferrocene; hydrogen In toluene at 80℃; under 38002.6 Torr; for 24h; Autoclave; optical yield given as %ee; enantioselective reaction; | 97% |
| With [bis(2-methylallyl)cycloocta-1,5-diene]ruthenium(II); C48H44Fe2P2; hydrogen; N,N,N',N'-tetramethylguanidine In 2-methyl-propan-1-ol at 80℃; for 4h; enantioselective reaction; | 97% |

| Conditions | Yield |
|---|---|
| With [Pd(IPr*IMe)An(3-Cl-pyridinyl)Cl2]; potassium carbonate; Trimethylacetic acid In N,N-dimethyl acetamide at 130℃; for 12h; regioselective reaction; | 96% |
-
-
92-71-7
2,5-diphenyloxazole

-
A
-
128600-19-1
4,5-Dimethoxy-2,5-diphenyl-2-oxazoline

-
B
-
126193-96-2
N-benzoyl-1,2,2-trimethoxy-2-phenylethyl amine

| Conditions | Yield |
|---|---|
| With bromine; potassium carbonate In methanol 1.) -78 deg C, 1 h; 2.) -15 - -5 deg C, 5 h; Yields of byproduct given; | A 95.5% B n/a |
-
-
92-71-7
2,5-diphenyloxazole

-
-
126193-96-2
N-benzoyl-1,2,2-trimethoxy-2-phenylethyl amine

| Conditions | Yield |
|---|---|
| With bromine; potassium carbonate In methanol 1.) -78 deg C, 1 h; 2.) -15 deg C, 3 d; | 95.5% |
| Multi-step reaction with 2 steps 1: 95.5 percent / K2CO3, Br2 / methanol / 1.) -78 deg C, 1 h; 2.) -15 - -5 deg C, 5 h 2: Br2, K2CO3 / methanol / 48 h / -10 °C View Scheme |

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In acetonitrile at 80℃; for 1h; Inert atmosphere; | 85% |
| With 2,2,6,6-tetramethyl-piperidine; bromine; sec.-butyllithium 1a) THF, dry ice bath, 0.5 h, b) ice cooling; Yield given. Multistep reaction; |
-
-
92-71-7
2,5-diphenyloxazole

-
-
189278-27-1
2-bromo-6-(trifluoromethyl)pyridine

| Conditions | Yield |
|---|---|
| With potassium acetate; palladium diacetate In N,N-dimethyl acetamide at 150℃; for 16h; Schlenk technique; | 85% |
-
-
92-71-7
2,5-diphenyloxazole

| Conditions | Yield |
|---|---|
| With dichloro(μ-chloro)(μ-hydrido)bis(η-p-cymene)diruthenium(II); hydrogen In 1,4-dioxane at 90℃; under 37503.8 Torr; for 40h; | 85% |
2,5-Diphenyloxazole Consensus Reports
2,5-Diphenyloxazole Specification
The 2,5-Diphenyloxazole, with the CAS registry number 92-71-7, is also known as Oxazole, 2,5-diphenyl-. It belongs to the product categories of Isoxazoles, Oxadiazoles, Oxazoles; Oxazole & Isoxazole. Its EINECS registry number is 202-181-3. This chemical's molecular formula is C15H11NO and molecular weight is 221.25. What's more, both its IUPAC name and systematic name are the same which is called 2,5-Diphenyl-1,3-oxazole. It should be stored in a cool and sealed place.
Physical properties about 2,5-Diphenyloxazole are: (1)ACD/LogP: 4.68; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 4.68; (4)ACD/LogD (pH 7.4): 4.68; (5)ACD/BCF (pH 5.5): 2120.62; (6)ACD/BCF (pH 7.4): 2120.64; (7)ACD/KOC (pH 5.5): 8369.09; (8)ACD/KOC (pH 7.4): 8369.15; (9)#H bond acceptors: 2; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 26.03 Å2; (13)Index of Refraction: 1.586; (14)Molar Refractivity: 65.828 cm3; (15)Molar Volume: 196.021 cm3; (16)Polarizability: 26.096×10-24 cm3; (17)Surface Tension: 43.05 dyne/cm; (18)Density: 1.129 g/cm3; (19)Flash Point: 162.302 °C; (20)Enthalpy of Vaporization: 58.18 kJ/mol; (21)Boiling Point: 359.998 °C at 760 mmHg; (22)Vapour Pressure: 0 mmHg at 25 °C.
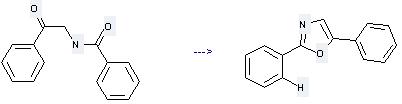
Preparation of 2,5-Diphenyloxazole: this chemical can be prepared by 2-Benzoylamino-1-phenyl-ethanone. This reaction needs reagent Burgess reagent and solvent tetrahydrofuran. The reaction time is 20 min. The yield is 93 %.
Uses of 2,5-Diphenyloxazole: (1) it is used as an organic scintillator; (2) it is used to produce other chemicals. For example, it can react with [1,2]oxathiolane 2,2-dioxide to get 3-(2,5-diphenyloxazolio-3)propanesulfonate. The reaction occurs with reagent 1,2-dichloro-benzene at temperature of 170 °C for 30 min. The yield is 70 %.
![2,5-Diphenyloxazole can react with [1,2]oxathiolane 2,2-dioxide to get 3-(2,5-diphenyloxazolio-3)propanesulfonate.](/UserFilesUpload/Uses of 2,5-Diphenyloxazole.jpg)
When you are dealing with this chemical, you should be very careful. This chemical is inflammation to the skin, eyes and respiratory system or other mucous membranes. Therefore, you should wear suitable protective clothing and gloves. And you must avoid contacting with skin and eyes. In case of contacting with eyes, you should rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1) SMILES: n1cc(oc1c2ccccc2)c3ccccc3
(2) InChI: InChI=1S/C15H11NO/c1-3-7-12(8-4-1)14-11-16-15(17-14)13-9-5-2-6-10-13/h1-11H
(3) InChIKey: CNRNYORZJGVOSY-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 750mg/kg (750mg/kg) | National Technical Information Service. Vol. AD277-689, |
Related Products
- 20,22-DIHYDRODIGITOXIN
- 20,29,30-Trinorlupane,(17alpha)-
- 20-ETHYL-6-β,8-DIHYDROXY-1-α-METHOXY-4-METHYLHETERATISAN-14-ONE
- 20-Ethylprostaglandin F2-alpha
- 20-Isopropylcholanthrene
- 20-METHYLCHOLANTHREN-15-ONE
- 20-METHYLCHOLANTHRENE PICRATE
- 20-METHYLCHOLANTHRENE-TRINITRO-BENZENE
- 20(S)-Ginsenoside C-K
- 2,10-DIFLUOROBENZO(rst)PENTAPHENE
- 927181-97-3
- 927181-98-4
- 927-18-4
- 927186-04-7
- 927186-53-6
- 927186-55-8
- 927186-60-5
- 927-20-8
- 927209-98-1
- 927-21-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View