-
Name
2,6-Dimethoxypyridine
- EINECS 228-334-4
- CAS No. 6231-18-1
- Article Data8
- CAS DataBase
- Density 1.064 g/cm3
- Solubility
- Melting Point
- Formula C7H9NO2
- Boiling Point 178.7 °C at 760 mmHg
- Molecular Weight 139.154
- Flash Point 61.7 °C
- Transport Information UN 1993
- Appearance clear colorless to light yellow liquid
- Safety 26-39-36/37/39-36
- Risk Codes 22-37/38-41-36/37/38-20/21/22
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn,  Xi
Xi
- Synonyms 2,6-Dimethoxypyridin;
- PSA 31.35000
- LogP 1.09880
Synthetic route

| Conditions | Yield |
|---|---|
| With sodium hydroxide | 94% |
| With 5-(di(adamantan-1-yl)phosphino)-1′,3′,5′-triphenyl-1′H-1,4′-bipyrazole; palladium diacetate; caesium carbonate In toluene at 80℃; for 24h; | 77% |
| With 5-(di-tert-butylphosphino)-1′, 3′, 5′-triphenyl-1′H-[1,4′]bipyrazole; tris-(dibenzylideneacetone)dipalladium(0); potassium hydroxide at 0.1℃; for 3h; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide for 8h; Reflux; | 94% |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 80℃; | 65% |

| Conditions | Yield |
|---|---|
| With sodium methylate In N,N-dimethyl-formamide at 80℃; for 2h; | 65% |
-
-
2402-78-0
2,6-dichloropyridine

-
-
124-41-4
sodium methylate

-
A
-
17228-64-7
6-chloro-2-methoxypyridine

-
B
-
6231-18-1
2,6-dimethoxypyridine

| Conditions | Yield |
|---|---|
| In methanol for 25h; Heating; | A 80 % Spectr. B 20 % Spectr. |


| Conditions | Yield |
|---|---|
| at 40℃; under 4500450 Torr; for 24h; Hilbert-Johnson reaction; | 100% |
| at 80℃; for 24h; | 72% |
-
-
6231-18-1
2,6-dimethoxypyridine

-
-
214360-56-2
3-iodo-2,6-dimethoxypyridine

| Conditions | Yield |
|---|---|
| Stage #1: 2,6-dimethoxypyridine With 2,2,6,6-tetramethyl-piperidine; n-butyllithium; zinc dichloro(N,N,N′,N′-tetramethylethylenediamine) In tetrahydrofuran; hexane at 20℃; for 2h; Inert atmosphere; Stage #2: With iodine In tetrahydrofuran; hexane at 20℃; Concentration; Time; Inert atmosphere; regioselective reaction; | 98% |
| With iodine; silver(I) acetate In acetonitrile at 23℃; for 0.5h; | 80% |
| With iodine; sodium hydrogencarbonate at 20℃; | 41% |
| With dichloro(N,N,N’,N‘-tetramethylethylenediamine)zinc; 2,2,6,6-tetramethylpiperidinyl-lithium In tetrahydrofuran at 20℃; for 2h; |
-
-
6231-18-1
2,6-dimethoxypyridine

-
-
13445-16-4
3-bromo-2,6-dimethoxypyridine

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In acetonitrile at 80℃; for 18h; | 97% |
| With N-Bromosuccinimide In acetonitrile for 10h; Reflux; | 89% |
| With N-Bromosuccinimide In acetonitrile for 10h; Heating; | 85% |

| Conditions | Yield |
|---|---|
| With (dppf)Ni(o-tol)Cl; caesium carbonate In neat (no solvent) at 130℃; for 16h; Inert atmosphere; Sealed tube; | 96% |

| Conditions | Yield |
|---|---|
| With C25H36O2P2Ru In tetrahydrofuran at 120℃; for 12h; Inert atmosphere; Sealed tube; | 96% |

| Conditions | Yield |
|---|---|
| In dichloromethane under Ar atm. to suspn. ZnBr2 in CH2Cl2 2 equiv. 2,6-dimethoxypyridine was added and stirred at room temp. for 12 h; solvent was removed under vac. at 40°C; elem. anal.; | 95% |
| In diethyl ether under Ar atm. to soln. ZnBr2 in ether 2,6-dimethoxypyridine was added; ppt. was washed with ether; elem. anal.; |
-
-
6231-18-1
2,6-dimethoxypyridine

-
-
4294-57-9
para-methylphenylmagnesium bromide

-
-
14435-88-2
2,6-di-p-tolylpyridine

| Conditions | Yield |
|---|---|
| With C68H72Cl2N6NiP2 In tetrahydrofuran at 25℃; for 24h; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| With ammonium peroxydisulfate; tris(2,2′-bipyrazine-N1,N1′)ruthenium(II) hexafluorophosphate In acetonitrile at 25℃; for 20h; Inert atmosphere; Irradiation; | 93% |
-
-
6231-18-1
2,6-dimethoxypyridine

-
-
609-65-4
o-chlorobenzoyl chloride

-
-
1335212-81-1
2-chlorophenyl(2,6-dimethoxypyridin-3-yl)ketone

| Conditions | Yield |
|---|---|
| Stage #1: 2,6-dimethoxypyridine With n-butyllithium; N,N,N,N,-tetramethylethylenediamine; 2,2,6,6-tetramethylpiperidinyl-lithium; copper(l) chloride In tetrahydrofuran; hexanes at 20℃; for 2h; Inert atmosphere; Stage #2: o-chlorobenzoyl chloride In tetrahydrofuran; hexanes at 0 - 20℃; for 16h; Inert atmosphere; | 91% |
| Stage #1: 2,6-dimethoxypyridine With N,N,N,N,-tetramethylethylenediamine; 2,2,6,6-tetramethylpiperidinyl-lithium; copper(l) chloride In tetrahydrofuran at 20℃; for 2h; Schlenk technique; Inert atmosphere; Stage #2: o-chlorobenzoyl chloride In tetrahydrofuran at 20℃; Schlenk technique; Inert atmosphere; | 91% |

| Conditions | Yield |
|---|---|
| In acetonitrile Reflux; | 90% |

| Conditions | Yield |
|---|---|
| With potassium hexamethylsilazane In tetrahydrofuran at 100℃; for 16h; Inert atmosphere; Schlenk technique; Sealed tube; Green chemistry; | 86% |

| Conditions | Yield |
|---|---|
| With ammonium peroxydisulfate; tris(2,2′-bipyrazine-N1,N1′)ruthenium(II) hexafluorophosphate In acetonitrile at 25℃; for 20h; Inert atmosphere; Irradiation; | 84% |

| Conditions | Yield |
|---|---|
| at 100℃; under 6000600 Torr; for 48h; Hilbert-Johnson reaction; | 83% |
-
-
6231-18-1
2,6-dimethoxypyridine

-
-
1352918-92-3
N-((4-chlorobenzyl)oxy)-N-methylacetamide

-
-
1352918-69-4
3-(4-chlorobenzyl)-2,6-dimethoxypyridine

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate In dichloromethane at 45℃; for 24h; Friedel Crafts alkylation; Inert atmosphere; | 83% |
-
-
6231-18-1
2,6-dimethoxypyridine

| Conditions | Yield |
|---|---|
| With N-chloro-succinimide; dimethyl sulfoxide In chloroform at 25℃; for 2h; Reagent/catalyst; Schlenk technique; | 83% |
| Multi-step reaction with 2 steps 1.1: N-Bromosuccinimide / acetonitrile / 10 h / Reflux 2.1: n-butyllithium; isopropylmagnesium chloride / tetrahydrofuran; hexane / 0.75 h / -2 - 0 °C / Inert atmosphere 2.2: 0 - 20 °C / Inert atmosphere View Scheme |
-
-
6231-18-1
2,6-dimethoxypyridine

-
-
65094-22-6
diethyl (bromodifluoromethyl)phosphonate

| Conditions | Yield |
|---|---|
| With potassium phosphate; fac-tris(2-phenylpyridinato-N,C2')iridium(III) In dichloromethane at 20℃; for 36h; Inert atmosphere; Irradiation; | 83% |

| Conditions | Yield |
|---|---|
| With CoIII(dimethylglyoximate)2(4-NMe2-C5H4N)Cl; 3,6‐di‐tert‐butyl‐9‐mesityl‐10‐phenylacridin‐10‐ium tetrafluoroborate In 1,2-dichloro-ethane at 20℃; for 24h; Heck Reaction; Irradiation; Inert atmosphere; regioselective reaction; | 83% |
-
-
6231-18-1
2,6-dimethoxypyridine

-
-
221006-70-8
2,6-dimethoxypyridine-3-boronic acid

| Conditions | Yield |
|---|---|
| Stage #1: 2,6-dimethoxypyridine With n-butyllithium; diisopropylamine In tetrahydrofuran; hexane at -78℃; for 3h; Stage #2: With Triisopropyl borate In tetrahydrofuran; hexane at -78℃; for 1h; Stage #3: With hydrogen bromide In water pH=6; | 82% |
| With n-butyllithium; Triisopropyl borate; diisopropylamine In tetrahydrofuran at -78 - 20℃; Inert atmosphere; | 80% |

| Conditions | Yield |
|---|---|
| With ammonium peroxydisulfate; tris(2,2′-bipyrazine-N1,N1′)ruthenium(II) hexafluorophosphate In acetonitrile at 25℃; for 20h; Inert atmosphere; Irradiation; | 82% |
-
-
6231-18-1
2,6-dimethoxypyridine

-
-
1365122-59-3
2,6-dimethoxy-3-(trifluoromethyl)pyridine

| Conditions | Yield |
|---|---|
| In acetonitrile Solvent; Irradiation; | 82% |
-
-
6231-18-1
2,6-dimethoxypyridine

-
-
16727-44-9
2,6-dimethoxy-3,5-dibromopyridine

| Conditions | Yield |
|---|---|
| With bromine In chloroform at 20℃; for 4h; | 80% |
-
-
6231-18-1
2,6-dimethoxypyridine

-
-
5419-55-6
Triisopropyl borate

-
-
1219744-53-2
2,6-dimethoxy-3-pyridinyl-boronic acid

| Conditions | Yield |
|---|---|
| Stage #1: 2,6-dimethoxypyridine With n-butyllithium; diisopropylamine In tetrahydrofuran at -78℃; for 3h; Inert atmosphere; Stage #2: Triisopropyl borate In tetrahydrofuran at -78 - 20℃; Inert atmosphere; | 80% |
-
-
6231-18-1
2,6-dimethoxypyridine

| Conditions | Yield |
|---|---|
| In hexane stirring a suspn. of tungsten complex and ligand in hexanes for 26 h; obtained as a mixt. of diastereomers; | 79% |
| In pentane to a soln. of complex and pyridine-compound was added pentane, the mixt.was stirred for 26 h; the mixt. was added to pentane, ppt. was dried in vac.; | 79% |
-
-
5672-90-2
N-(trifluoromethyl)acyloxyphthalimide

-
-
6231-18-1
2,6-dimethoxypyridine

-
-
848827-76-9
2,6-dimethoxy-3-(N-phthalimido)pyridine

| Conditions | Yield |
|---|---|
| With tris[2-phenylpyridinato-C2,N]iridium(III) In acetonitrile at 20℃; for 24h; Sealed tube; Irradiation; | 79% |

| Conditions | Yield |
|---|---|
| With sodium hydride; lithium iodide In tetrahydrofuran at 0 - 60℃; for 5h; Inert atmosphere; Sealed tube; | 79% |
-
-
6231-18-1
2,6-dimethoxypyridine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 3.5h; Glovebox; Inert atmosphere; | 78.6% |

| Conditions | Yield |
|---|---|
| Stage #1: dibutylamine With n-butyllithium In tetrahydrofuran; hexane at -78 - 20℃; for 0.166667h; Inert atmosphere; Schlenk technique; Stage #2: 2,6-dimethoxypyridine In tetrahydrofuran; hexane at 60℃; for 0.166667h; Inert atmosphere; Schlenk technique; | 78% |
-
-
6231-18-1
2,6-dimethoxypyridine

-
A
-
13445-16-4
3-bromo-2,6-dimethoxypyridine

-
B
-
16727-44-9
2,6-dimethoxy-3,5-dibromopyridine

| Conditions | Yield |
|---|---|
| With bromine In tetrachloromethane at -40 - -30℃; | A 76% B n/a |
| With pyridinium hydrobromide perbromide In tetrachloromethane Rt, overnight, then reflux, 2 h; | A 30% B 15% |
| With potassium bromide In water; dimethyl sulfoxide at 25℃; Irradiation; Overall yield = 69 percent; |
2,6-Dimethoxypyridine Chemical Properties
The density of 2,6-Dimethoxypyridine(6231-18-1) is 1.053 g/mL at 25 °C(lit.) and it has a boiling point of 178-180 °C(lit.). The refractive index is about 1.503(lit.). Its flash point is 143 °F.
The chemical synonyms of 2,6-Dimethoxypyridine(6231-18-1) are 2,6-DIMETHOXYPYRIDINE;2,6-Dimethoxypyridine, 98+%;2,6-Dimethoxypyridine,99%
The molecular structure of 2,6-Dimethoxypyridine(6231-18-1):
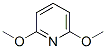
2,6-Dimethoxypyridine Toxicity Data With Reference
LD50/LC50: RTECS: Not available.
Carcinogenicity: 2,6-Dimethoxypyridine - Not listed as a carcinogen by ACGIH, IARC, NTP, or CA Prop 65.
Other: The toxicological properties have not been fully investigated.
2,6-Dimethoxypyridine Safety Profile
Hazard Codes Xn,Xi
Risk Statements 22-37/38-41-36/37/38-20/21/22
Safety Statements 26-39-36/37/39-36
RIDADR 1993
WGK Germany 3
HazardClass 3.2
PackingGroup III
2,6-Dimethoxypyridine Specification
Conditions to Avoid: Incompatible materials.
Incompatibilities with Other Materials Strong oxidizing agents, strong acids.
Hazardous Decomposition Products Nitrogen oxides, carbon monoxide, carbon dioxide.
Hazardous Polymerization Will not occur.
Related Products
- 20,22-DIHYDRODIGITOXIN
- 20,29,30-Trinorlupane,(17alpha)-
- 20-ETHYL-6-β,8-DIHYDROXY-1-α-METHOXY-4-METHYLHETERATISAN-14-ONE
- 20-Ethylprostaglandin F2-alpha
- 20-Isopropylcholanthrene
- 20-METHYLCHOLANTHREN-15-ONE
- 20-METHYLCHOLANTHRENE PICRATE
- 20-METHYLCHOLANTHRENE-TRINITRO-BENZENE
- 20(S)-Ginsenoside C-K
- 2,10-DIFLUOROBENZO(rst)PENTAPHENE
- 623-12-1
- 623-13-2
- 623152-17-0
- 623-15-4
- 62316-07-8
- 62316-48-7
- 62316-68-1
- 62-31-7
- 623172-56-5
- 623-17-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View