-
Name
2-Acetylfluorene
- EINECS 212-310-5
- CAS No. 781-73-7
- Article Data45
- CAS DataBase
- Density 1.157 g/cm3
- Solubility water: 1 g/L (20 °C)
- Melting Point 128-129 °C(lit.)
- Formula C15H12O
- Boiling Point 381.4 °C at 760 mmHg
- Molecular Weight 208.26
- Flash Point 169 °C
- Transport Information
- Appearance light yellow crystalline powder
- Safety 24/25
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Ketone,fluoren-2-yl methyl (6CI,7CI,8CI);1-(9H-Fluoren-2-yl)ethanone;2-Acetyl-9H-fluorene;2-Fluorenyl methylketone;NSC 12351;
- PSA 17.07000
- LogP 3.46040
Synthetic route

| Conditions | Yield |
|---|---|
| trifluoroacetic acid for 10h; Heating; | A 44% B 99% |

-
-
228263-51-2
2-(4-acetylphenyl)benzyl chloride

-
-
781-73-7
1-(9H-fluoren-2-yl)ethanone

| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; caesium carbonate; Trimethylacetic acid In tetrahydrofuran at 25℃; for 18h; Glovebox; Schlenk technique; Inert atmosphere; | 94% |
| With palladium diacetate; caesium carbonate; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl In 1,2-dimethoxyethane at 100℃; for 12h; Inert atmosphere; | 48% |

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid for 5h; Heating; | 89% |

| Conditions | Yield |
|---|---|
| With N-trifluoroacetylimidazole; trifluoroacetic acid for 10h; Heating; | 82% |
| With pyridin-2-yl trifluoromethanesulfonate; trifluoroacetic acid for 5h; Heating; | 81% |

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid for 5h; Heating; | 80% |

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride In 1,2-dichloro-ethane at 0℃; for 1h; Friedel Crafts acylation; | 79% |
| With carbon disulfide; aluminium trichloride | |
| With aluminium trichloride; nitrobenzene |
-
-
7446-70-0
aluminium trichloride

-
-
86-73-7
9H-fluorene

-
-
108-24-7
acetic anhydride

-
-
98-95-3
nitrobenzene

-
-
781-73-7
1-(9H-fluoren-2-yl)ethanone

| Conditions | Yield |
|---|---|
| anfangs unter Kuehlung; | |
| anfangs unter Kuehlung; |

-
-
75-15-0
carbon disulfide

-
-
7446-70-0
aluminium trichloride

-
-
86-73-7
9H-fluorene

-
-
75-36-5
acetyl chloride

-
-
781-73-7
1-(9H-fluoren-2-yl)ethanone

| Conditions | Yield |
|---|---|
| at 5 - 10℃; |

-
-
7446-70-0
aluminium trichloride

-
-
86-73-7
9H-fluorene

-
-
98-95-3
nitrobenzene

-
-
75-36-5
acetyl chloride

-
-
781-73-7
1-(9H-fluoren-2-yl)ethanone

| Conditions | Yield |
|---|---|
| at 5 - 10℃; |

-
-
7446-70-0
aluminium trichloride

-
-
86-73-7
9H-fluorene

-
-
108-24-7
acetic anhydride

-
-
781-73-7
1-(9H-fluoren-2-yl)ethanone

| Conditions | Yield |
|---|---|
| anfangs unter Kuehlung; |


| Conditions | Yield |
|---|---|
| With bromine In acetic acid | A n/a B 77.0 g (74 wt.% as calculated for fluorene) |
-
-
38580-83-5
2-phenylbenzyl chloride

-
-
228263-51-2
2-(4-acetylphenyl)benzyl chloride

-
A
-
86-73-7
9H-fluorene

-
B
-
781-73-7
1-(9H-fluoren-2-yl)ethanone

| Conditions | Yield |
|---|---|
| With palladium diacetate; caesium carbonate; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl In 1,2-dimethoxyethane at 100℃; for 3h; Inert atmosphere; |


-
-
781-73-7
1-(9H-fluoren-2-yl)ethanone

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water |
-
-
31477-49-3
1-(2'-aminobiphenyl-4-yl)ethanone

-
-
781-73-7
1-(9H-fluoren-2-yl)ethanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: hydrogenchloride; sodium nitrite / water / 1 h / Cooling 1.2: 20 °C 2.1: palladium diacetate; potassium hydrogencarbonate; potassium acetate; isopropyl alcohol / water; N,N-dimethyl-formamide; N,N-dimethyl acetamide / 10 h / 75 °C / Schlenk technique; Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: potassium carbonate; palladium diacetate / acetone; water / 65 °C / Sealed tube; Inert atmosphere 2.1: hydrogenchloride; sodium nitrite / water / 1 h / Cooling 2.2: 20 °C 3.1: palladium diacetate; potassium hydrogencarbonate; potassium acetate; isopropyl alcohol / water; N,N-dimethyl-formamide; N,N-dimethyl acetamide / 10 h / 75 °C / Schlenk technique; Inert atmosphere View Scheme |
-
-
149104-90-5
4-acetylphenylboronic acid

-
-
781-73-7
1-(9H-fluoren-2-yl)ethanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: potassium carbonate; palladium diacetate / acetone; water / 65 °C / Sealed tube; Inert atmosphere 2.1: hydrogenchloride; sodium nitrite / water / 1 h / Cooling 2.2: 20 °C 3.1: palladium diacetate; potassium hydrogencarbonate; potassium acetate; isopropyl alcohol / water; N,N-dimethyl-formamide; N,N-dimethyl acetamide / 10 h / 75 °C / Schlenk technique; Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1: palladium diacetate; potassium phosphate; johnphos / tetrahydrofuran / 50 °C / Inert atmosphere 2: triethylamine; methanesulfonyl chloride / dichloromethane / 0 - 25 °C 3: bis-triphenylphosphine-palladium(II) chloride; caesium carbonate; Trimethylacetic acid / tetrahydrofuran / 18 h / 25 °C / Glovebox; Schlenk technique; Inert atmosphere View Scheme |

-
-
781-73-7
1-(9H-fluoren-2-yl)ethanone

| Conditions | Yield |
|---|---|
| With β-lactoglobulin from milk In aq. phosphate buffer; ethanol at 37℃; for 96h; pH=7.4; Catalytic behavior; Darkness; Enzymatic reaction; |
-
-
18982-54-2
o-bromobenzyl alcohol

-
-
781-73-7
1-(9H-fluoren-2-yl)ethanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: palladium diacetate; potassium phosphate; johnphos / tetrahydrofuran / 50 °C / Inert atmosphere 2: triethylamine; methanesulfonyl chloride / dichloromethane / 0 - 25 °C 3: bis-triphenylphosphine-palladium(II) chloride; caesium carbonate; Trimethylacetic acid / tetrahydrofuran / 18 h / 25 °C / Glovebox; Schlenk technique; Inert atmosphere View Scheme |

-
-
781-73-7
1-(9H-fluoren-2-yl)ethanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: triethylamine; methanesulfonyl chloride / dichloromethane / 0 - 25 °C 2: bis-triphenylphosphine-palladium(II) chloride; caesium carbonate; Trimethylacetic acid / tetrahydrofuran / 18 h / 25 °C / Glovebox; Schlenk technique; Inert atmosphere View Scheme |
-
-
781-73-7
1-(9H-fluoren-2-yl)ethanone

-
-
110827-07-1
2-Acetylfluorene oxime

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride; sodium acetate In ethanol; water at 95℃; | 100% |
| With hydroxylamine hydrochloride; sodium acetate In ethanol; water at 100℃; | |
| With pyridine; hydroxylamine hydrochloride In ethanol at 60℃; for 1h; | |
| With hydroxylamine hydrochloride; sodium acetate In ethanol; water | |
| With hydroxylamine hydrochloride; sodium acetate In ethanol at 90℃; for 2h; Sealed tube; Inert atmosphere; |
-
-
781-73-7
1-(9H-fluoren-2-yl)ethanone

-
-
110827-07-1
1-fluoren-2-yl-ethanone oxime

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride; sodium acetate In water for 1h; Heating; | 99% |
-
-
781-73-7
1-(9H-fluoren-2-yl)ethanone

| Conditions | Yield |
|---|---|
| With bis(1,5-cyclooctadiene)diiridium(I) dichloride; C53H73FeN2O2PS; hydrogen; lithium tert-butoxide In isopropyl alcohol at 25 - 30℃; under 22801.5 Torr; for 12h; Autoclave; enantioselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With sodium dichromate In acetic acid Oxidation; Heating; | 98.5% |
| With potassium hydroxide; water; bromine at 90℃; for 0.5h; | 78% |
| With sodium dichromate; acetic anhydride In acetic acid for 2h; Heating; | 56% |
| With potassium hypochlorite | |
| With sodium hydroxide; sodium hypochlorite |
-
-
781-73-7
1-(9H-fluoren-2-yl)ethanone

-
-
123-11-5
4-methoxy-benzaldehyde

-
-
197250-39-8
3-(9H-fluoren-2-yl)-3-oxopropanenitrile

| Conditions | Yield |
|---|---|
| With ammonium acetate; acetic acid Reflux; | 98% |

-
-
781-73-7
1-(9H-fluoren-2-yl)ethanone

-
-
39696-13-4
2-bromo-1-(9H-fluoren-2-yl)ethanone

| Conditions | Yield |
|---|---|
| With bromine In 1,4-dioxane; diethyl ether for 0.5h; | 97% |
| With diethyl ether; bromine at 0℃; | |
| With chloroform; hydrogen bromide; bromine; acetic acid |
-
-
781-73-7
1-(9H-fluoren-2-yl)ethanone

-
-
100-52-7
benzaldehyde

-
-
197250-39-8
3-(9H-fluoren-2-yl)-3-oxopropanenitrile

| Conditions | Yield |
|---|---|
| With ammonium acetate; acetic acid Reflux; | 97% |

-
-
781-73-7
1-(9H-fluoren-2-yl)ethanone

-
-
99-61-6
3-nitro-benzaldehyde

-
-
126-81-8
dimedone

-
-
1446491-13-9
2-(9H-fluoren-2-yl)-7,8-dihydro-7,7-dimethyl-4-(3-nitrophenyl)quinolin-5(6H)-one

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; ammonium carbonate In water at 20℃; Green chemistry; | 96% |

-
-
781-73-7
1-(9H-fluoren-2-yl)ethanone

-
-
106-95-6
allyl bromide

-
-
1015254-28-0
2-(9H-fluoren-7-yl)pent-4-en-2-ol

| Conditions | Yield |
|---|---|
| Stage #1: 1-(9H-fluoren-2-yl)ethanone; allyl bromide With magnesium at 20℃; for 0.116667h; Neat (no solvent); Stage #2: With ammonium chloride Saturated solution; | 95% |
| With copper; zinc at 20℃; for 0.216667h; Barbier-type reaction; | 93% |
-
-
781-73-7
1-(9H-fluoren-2-yl)ethanone

-
-
126-81-8
dimedone

-
-
1122-91-4
4-bromo-benzaldehyde

-
-
1446491-12-8
4-(4-bromophenyl)-2-(9H-fluoren-2-yl)-7,8-dihydro-7,7-dimethylquinolin-5(6H)-one

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; ammonium carbonate In water at 20℃; Green chemistry; | 95% |

-
-
781-73-7
1-(9H-fluoren-2-yl)ethanone

-
-
104-88-1
4-chlorobenzaldehyde

-
-
126-81-8
dimedone

-
-
1446491-11-7
4-(4-chlorophenyl)-2-(9H-fluoren-2-yl)-7,8-dihydro-7,7-dimethylquinolin-5(6H)-one

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; ammonium carbonate In water at 20℃; Green chemistry; | 94% |
| With ammonium acetate; oxygen In ethanol for 4h; Catalytic behavior; Reflux; | 94% |


| Conditions | Yield |
|---|---|
| With ammonium acetate; acetic acid Reflux; | 94% |


| Conditions | Yield |
|---|---|
| With ammonium acetate; acetic acid Reflux; | 94% |

-
-
606-23-5
1H-indene-1,3(2H)-dione

-
-
781-73-7
1-(9H-fluoren-2-yl)ethanone

-
-
104-88-1
4-chlorobenzaldehyde

-
-
1221745-70-5
4-(4-chlorophenyl)-2-(9H-fluoren-2-yl)-indeno[1,2-b]pyridin-5-one

| Conditions | Yield |
|---|---|
| With ammonium acetate; L-proline In ethanol at 20℃; for 2.5h; | 93% |


| Conditions | Yield |
|---|---|
| With 3-[(pyridin-3-yl)methyl]mercaptopropylsilica In ethanol; water for 1.08333h; Reflux; diastereoselective reaction; | 93% |


| Conditions | Yield |
|---|---|
| With triethylamine In water at 100℃; for 4h; | 93% |

-
-
606-23-5
1H-indene-1,3(2H)-dione

-
-
781-73-7
1-(9H-fluoren-2-yl)ethanone

-
-
1122-91-4
4-bromo-benzaldehyde

-
-
1221745-69-2
4-(4-bromophenyl)-2-(9H-fluoren-2-yl)-indeno[1,2-b]pyridin-5-one

| Conditions | Yield |
|---|---|
| With ammonium acetate; L-proline In ethanol at 20℃; for 2.5h; | 92% |


| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; iodine In water; dimethyl sulfoxide at 110℃; | 92% |

| Conditions | Yield |
|---|---|
| With triethylamine In water at 100℃; for 3h; | 92% |


| Conditions | Yield |
|---|---|
| With sodium hypophosphite monohydrate; 5%-palladium/activated carbon; hypophosphorous acid In water at 100℃; | 91% |
| Wolff-Kishner reduction; | 80% |
| With potassium hydroxide; hydrazine In ethylene glycol 1.) 130-140 deg C 2 h, 2.) reflux 4 h; | 75% |
-
-
781-73-7
1-(9H-fluoren-2-yl)ethanone

-
-
42136-05-0
2-acetylfluoren-9-one

| Conditions | Yield |
|---|---|
| With copper(II) choride dihydrate; 4,7-bis(2-(2-(2-methoxyethoxy)ethoxy)ethoxy)-1,10-phenanthroline; oxygen; sodium hydroxide In water at 50℃; under 2250.23 Torr; for 16h; Schlenk technique; | 91% |
| With C30H44N2O8; oxygen; iron(II) sulfate; sodium fluoride In water at 100℃; under 1500.15 Torr; for 9h; | 91% |
| With oxygen; lithium hexamethyldisilazane In tetrahydrofuran at 60℃; under 760.051 Torr; for 12h; Sealed tube; Green chemistry; chemoselective reaction; | 87% |
-
-
781-73-7
1-(9H-fluoren-2-yl)ethanone

-
-
56522-24-8
tert-butyldimethylsilyl cyanide

-
-
1365529-71-0
C22H27NOSi

| Conditions | Yield |
|---|---|
| With tin ion-exchanged montmorillonite In dichloromethane at 25℃; for 0.25h; Inert atmosphere; | 91% |

| Conditions | Yield |
|---|---|
| With sodium hydrogen sulfate monohydrate; dihydrogen peroxide In water; toluene at 60℃; for 24h; | 91% |

| Conditions | Yield |
|---|---|
| With ammonium acetate; acetic acid Reflux; | 91% |

-
-
781-73-7
1-(9H-fluoren-2-yl)ethanone

| Conditions | Yield |
|---|---|
| With C37H40MnN2O2P2(1+)*Br(1-); sodium t-butanolate In isopropyl alcohol at 50℃; for 3h; Inert atmosphere; Schlenk technique; enantioselective reaction; | 91% |
-
-
109-65-9
1-bromo-butane

-
-
781-73-7
1-(9H-fluoren-2-yl)ethanone

-
-
883751-49-3
1-(9,9-di-n-butylfluorene-2-yl)-ethanone

| Conditions | Yield |
|---|---|
| Stage #1: 1-(9H-fluoren-2-yl)ethanone With potassium iodide; potassium hydroxide In dimethyl sulfoxide at 15℃; Inert atmosphere; Stage #2: 1-bromo-butane In dimethyl sulfoxide at 15℃; for 3h; | 90.8% |
-
-
781-73-7
1-(9H-fluoren-2-yl)ethanone

-
-
106-96-7
propargyl bromide

-
-
1193092-71-5
2-(9H-fluoren-7-yl)pent-4-yn-2-ol

| Conditions | Yield |
|---|---|
| Stage #1: 1-(9H-fluoren-2-yl)ethanone; propargyl bromide With zinc/copper couple at -16 - -14℃; for 0.5h; Barbier type reaction; Neat (no solvent); Stage #2: With ammonium chloride regioselective reaction; | 90% |
-
-
606-23-5
1H-indene-1,3(2H)-dione

-
-
781-73-7
1-(9H-fluoren-2-yl)ethanone

-
-
100-52-7
benzaldehyde

-
-
1221745-73-8
4-phenyl-2-(9H-fluoren-2-yl)-indeno[1,2-b]pyridin-5-one

| Conditions | Yield |
|---|---|
| With ammonium acetate; L-proline In ethanol at 20℃; for 3h; | 90% |

2-Acetylfluorene Chemical Properties
IUPAC Name: 2-Acetylfluorene
The MF of 2-Acetylfluorene (781-73-7) is C15H12O.
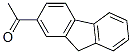
The MW of 2-Acetylfluorene (781-73-7) is 208.26.
Synonyms of 2-Acetylfluorene (781-73-7): 1-(9H-fluoren-2-yl)ethan-1-one ; Ethanone, 1- (9H-fluoren-2-yl)- ; 2-Acetofluorene ; 2-Fluorenyl methyl ketone
Product Categories: Fluorene Derivatives;Fluorenes, Flurenones;Fluorenes;Fluorenes & Fluorenones;C15 to C38;Carbonyl Compounds;Ketones
Form: light yellow crystalline powder
Index of Refraction: 1.627
EINECS: 212-310-5
Density: 1.157 g/ml
Flash Point: 169 °C
Boiling Point: 381.4 °C
Melting Point: 128-129 °C
Water Solubility: 1 g/L (20 ºC)
BRN: 1871711
2-Acetylfluorene Uses
2-Acetylfluorene (781-73-7) is used in organic synthesis.
2-Acetylfluorene Production
In the presence of anhydrous aluminum chloride, fluorene with acetic anhydride occurs Friedel-Crafts reaction of 2 - acetyl-fluorene.
2-Acetylfluorene Toxicity Data With Reference
| 1. | dnr-esc 80 mg/L | MUREAV Mutation Research. 119 (1983),135. |
2-Acetylfluorene Safety Profile
Mutation data reported. A flammable liquid. When heated to decomposition it emits acrid smoke and irritating vapors.
Safety information of 2-Acetylfluorene (781-73-7):
Safety Statements
24/25 Avoid contact with skin and eyes
WGK Germany 3
RTECS OB3800000
2-Acetylfluorene Standards and Recommendations
DOT Classification: 3; Label: Flammable Liquid
2-Acetylfluorene Specification
Store in a cool, dry place. Keep container closed when not in use.Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation.
Related Products
- 2-Acetylfluorene
- 78177-12-5
- 78180-08-2
- 78181-14-3
- 78-18-2
- 78182-00-0
- 78183-34-3
- 78183-55-8
- 78186-34-2
- 78190-05-3
- 78190-11-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View