-
Name
2-Amino-3-picoline
- EINECS 216-501-4
- CAS No. 1603-40-3
- Article Data20
- CAS DataBase
- Density 1.068 g/cm3
- Solubility soluble in water
- Melting Point 29-31 °C(lit.)
- Formula C6H8N2
- Boiling Point 220.9 °C at 760 mmHg
- Molecular Weight 108.143
- Flash Point 109.9 °C
- Transport Information UN 2811 6.1/PG 3
- Appearance clear yellow liquid after melting
- Safety 36/37/39-45-37/39-28A-26
- Risk Codes 23/24/25-33-36/37/38-25
-
Molecular Structure
-
Hazard Symbols
 T
T
- Synonyms 3-Picoline,2-amino- (7CI,8CI);2-Amino-b-picoline;3-Methyl-2-aminopyridine;3-Methyl-2-pyridinamine;3-Methylpyridin-2-ylamine;NSC 176169;NSC 450;3-Methyl-pyridin-2-ylamine;
- PSA 38.91000
- LogP 1.55340
Synthetic route

| Conditions | Yield |
|---|---|
| With copper(I) oxide; potassium carbonate; ammonium hydroxide; N,N-dimethylethylenediamine In ethylene glycol at 60℃; for 16h; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene; toluene-4-sulfonic acid In 1,2-dichloro-ethane at 20℃; for 1h; Schlenk technique; chemoselective reaction; | A n/a B 96% |
-
-
937648-82-3
3-methyl-2-pyridinecarboxamide

-
-
1603-40-3
3-methylpyridin-2-ylamine

| Conditions | Yield |
|---|---|
| With sodium hypobromide; water; sodium hydroxide at 0 - 60℃; for 0.5h; Hofmann Rearrangement; | 87.8% |
-
-
553626-46-3
N-cyclohexyl-2-isopropyl-8-methylimidazo[1,2-a]pyridin-3-amine

-
A
-
1603-40-3
3-methylpyridin-2-ylamine

-
B
-
32998-89-3
N-cyclohexyl-3-methyl-2-oxobutanamide

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene; toluene-4-sulfonic acid In 1,2-dichloro-ethane at 20℃; for 1h; Schlenk technique; chemoselective reaction; | A n/a B 74% |

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene; toluene-4-sulfonic acid In 1,2-dichloro-ethane at 20℃; for 1h; Schlenk technique; chemoselective reaction; | A n/a B 72% |

| Conditions | Yield |
|---|---|
| With acetamidine hydrochloride; sodium hydroxide In water; dimethyl sulfoxide at 130℃; for 24h; Schlenk technique; chemoselective reaction; | 43% |
-
-
108-99-6
3-Methylpyridine

-
A
-
1603-41-4
(5-methyl-pyridin-2-yl)amine

-
B
-
1603-40-3
3-methylpyridin-2-ylamine

| Conditions | Yield |
|---|---|
| With sodium amide; xylene | |
| With ammonia; sodium; ethanolamine In toluene at 60 - 170℃; under 22502.3 - 41254.1 Torr; Pressure; Temperature; Autoclave; Inert atmosphere; Large scale; | A 1928 g B 144 g |

| Conditions | Yield |
|---|---|
| With sodium amide; xylene at 135 - 140℃; | |
| With sodium amide In o-xylene Chichibabin reaction; |

-
-
1603-40-3
3-methylpyridin-2-ylamine

| Conditions | Yield |
|---|---|
| With hydrogenchloride Hydrolysis; |
-
-
1003-73-2
3-picoline-N-oxide

-
A
-
1603-41-4
(5-methyl-pyridin-2-yl)amine

-
B
-
1603-40-3
3-methylpyridin-2-ylamine

| Conditions | Yield |
|---|---|
| Stage #1: 3-picoline-N-oxide With p-toluenesulfonylanhydride; tert-butylamine In various solvent(s) for 0.25h; Stage #2: With trifluoroacetic acid In various solvent(s) at 70℃; Further stages. Title compound not separated from byproducts.; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: CH2Cl2 / Heating 2: conc. HCl View Scheme |
-
-
18368-76-8
2 chloro-3-methylpyridine

-
-
1603-40-3
3-methylpyridin-2-ylamine

| Conditions | Yield |
|---|---|
| With bis(η3-allyl-μ-chloropalladium(II)); 2-[di-(3S,5S,7S)-adamantan-1-ylphosphino]-N,N-dimethylaniline; ammonia; sodium t-butanolate In 1,4-dioxane at 110 - 120℃; Buchwald-Hartwig amination; Inert atmosphere; |
-
-
18368-76-8
2 chloro-3-methylpyridine

-
A
-
1603-40-3
3-methylpyridin-2-ylamine

-
B
-
6654-69-9
3,3’-dimethyl-2,2’-dipyridylamine

| Conditions | Yield |
|---|---|
| With diphenyl(2',4',6'-triisopropyl-4-methoxy-3,5,6-trimethyl-[1,1'-biphenyl]-2-yl)phosphine; diphenyl(2',4',6'-triisopropyl-5-methoxy-3,4,6-trimethyl-[1,1'-biphenyl]-2-yl)phosphine; C50H58NO4PPdS; C50H58NO4PPdS; ammonia; sodium t-butanolate In 1,4-dioxane at 50℃; for 24h; Inert atmosphere; |

-
A
-
1603-40-3
3-methylpyridin-2-ylamine

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water for 4h; |

-
A
-
1603-40-3
3-methylpyridin-2-ylamine

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water for 4h; |

| Conditions | Yield |
|---|---|
| With dodecacarbonyl-triangulo-triruthenium; isopropyl alcohol at 140℃; for 24h; Inert atmosphere; Sealed tube; | A 22 %Spectr. B 58 %Spectr. C 14 %Spectr. |


| Conditions | Yield |
|---|---|
| Stage #1: 3-methylpyridin-2-ylamine With bromine In dichloromethane at 0 - 20℃; for 4.66667h; Stage #2: With sodium hydroxide In dichloromethane; water pH=9; | 100% |
| With 1-butyl-3-methylpyridinium tribromide at 20℃; | 97% |
| With bromine; iodine; acetic acid at 0 - 10℃; Reagent/catalyst; Temperature; | 95.2% |
-
-
1603-40-3
3-methylpyridin-2-ylamine

| Conditions | Yield |
|---|---|
| With hydrogenchloride; hydrogen; platinum(IV) oxide In ethanol; water under 2068.65 Torr; for 6.5h; | 100% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene at 110℃; for 17h; Dean-Stark; | 100% |

| Conditions | Yield |
|---|---|
| With pyridine; tert.-butylhydroperoxide; iodine In acetonitrile at 100℃; for 12h; Green chemistry; | 100% |
| With sodium iodide dichloride In water; N,N-dimethyl-formamide at 80℃; for 1.5h; | 76% |
| With sodium iodine dichloride In water; N,N-dimethyl-formamide at 80℃; for 1.5h; | 76% |
| With copper(II) nitrate In acetonitrile at 85℃; for 1h; Inert atmosphere; regioselective reaction; | 1 g |
-
-
1603-40-3
3-methylpyridin-2-ylamine

-
-
675-10-5
4-hydroxy-6-methyl-2-pyron

-
-
61066-33-9, 61066-34-0, 61066-35-1, 61127-23-9
pyrimidine-2,4,5,6(1H,3H)-tetraone

-
-
1367748-01-3
2-amino-3-methylpyridinium 3-(5-hydroxy-2,4,6-trioxohexahydropyrimidin-5-yl)-6-methyl-2,4-dioxo-3,4-dihydro-2H-pyran-3-ide

| Conditions | Yield |
|---|---|
| In chloroform for 3h; Reflux; | 99% |

-
-
1603-40-3
3-methylpyridin-2-ylamine

-
-
1076-38-6
4-hydroxy[1]benzopyran-2-one

-
-
61066-33-9, 61066-34-0, 61066-35-1, 61127-23-9
pyrimidine-2,4,5,6(1H,3H)-tetraone

-
-
1367747-98-5
2-amino-3-methylpyridinium 3-(5-hydroxy-2,4,6-trioxohexahydropyrimidin-5-yl)-2,4-dioxochroman-3-ide

| Conditions | Yield |
|---|---|
| In chloroform for 3h; Reflux; | 99% |

-
-
1603-40-3
3-methylpyridin-2-ylamine

-
-
61066-33-9, 61066-34-0, 61066-35-1, 61127-23-9
pyrimidine-2,4,5,6(1H,3H)-tetraone

-
-
83-72-7
2-Hydroxy-1,4-naphthoquinone

-
-
1367748-05-7
2-amino-3-methylpyridinium 2-(5-hydroxy-2,4,6-trioxohexahydropyrimidin-5-yl)-1,3,4-trioxo-1,2,3,4-tetrahydronaphthalen-2-ide

| Conditions | Yield |
|---|---|
| In chloroform for 1h; Reflux; | 99% |


| Conditions | Yield |
|---|---|
| In chloroform at 20℃; for 1h; | 99% |
-
-
1603-40-3
3-methylpyridin-2-ylamine

-
-
2032-35-1
Bromoacetaldehyde diethyl acetal

-
-
874-10-2
8-methylimidazo[1,2-a]pyridine

| Conditions | Yield |
|---|---|
| With hydrogen bromide In ethanol; water at 90℃; for 26h; | 98% |
| With sodium carbonate In 1,4-dioxane; water for 22h; Cyclization; Tschitschibabin reaction; Heating; | 20% |

| Conditions | Yield |
|---|---|
| With 4 A molecular sieve In benzene at 80℃; for 5h; | 98% |

| Conditions | Yield |
|---|---|
| With 4 A molecular sieve In benzene at 80℃; for 5h; | 98% |
-
-
1603-40-3
3-methylpyridin-2-ylamine

-
-
61066-33-9, 61066-34-0, 61066-35-1, 61127-23-9
pyrimidine-2,4,5,6(1H,3H)-tetraone

-
-
769-42-6
1,3-dimethylbarbituric acid

-
-
1367748-03-5
2-amino-3-methylpyridinium 5-(5-hydroxy-2,4,6-trioxohexahydropyrimidin-5-yl)-1,3-dimethyl-2,4,6-trioxohexahydropyrimidin-5-ide

| Conditions | Yield |
|---|---|
| In chloroform for 4h; Reflux; | 98% |

-
-
1603-40-3
3-methylpyridin-2-ylamine

-
-
105-45-3
acetoacetic acid methyl ester

-
-
30247-65-5
2,9-dimethyl-4H-pyrido[1,2-a]pyrimidin-4-one

| Conditions | Yield |
|---|---|
| With bismuth(III) chloride at 100℃; for 3h; Green chemistry; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-methylpyridin-2-ylamine; phenylglyoxal hydrate In 1,2-dichloro-benzene at 20℃; Sealed tube; Stage #2: Cyclopropylacetylene With copper(l) iodide In 1,2-dichloro-benzene at 120℃; for 5h; Sealed tube; regioselective reaction; | 98% |


| Conditions | Yield |
|---|---|
| In toluene at 100℃; for 0.25h; | 97.8% |
-
-
1603-40-3
3-methylpyridin-2-ylamine

-
-
15687-27-1
ibuprofen

| Conditions | Yield |
|---|---|
| Stage #1: ibuprofen With 1,1'-carbonyldiimidazole In dichloromethane at 20℃; for 0.5h; Stage #2: 3-methylpyridin-2-ylamine In dichloromethane for 72h; Heating; | 97% |
-
-
1603-40-3
3-methylpyridin-2-ylamine

-
-
98-86-2
acetophenone

-
-
660432-29-1
3-iodo-8-methyl-2-phenylimidazo[1,2-a]pyridine

| Conditions | Yield |
|---|---|
| With iodine; oxygen In 1,2-dichloro-benzene at 100℃; for 20h; | 97% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-methylpyridin-2-ylamine; phenylglyoxal hydrate In 1,2-dichloro-benzene at 20℃; Sealed tube; Stage #2: n-octyne With copper(l) iodide In 1,2-dichloro-benzene at 120℃; for 5h; Sealed tube; regioselective reaction; | 97% |

-
-
1603-40-3
3-methylpyridin-2-ylamine

-
-
105-53-3
diethyl malonate

-
-
17326-09-9
2-hydroxy-9-methyl-4H-pyrido[1,2-a]pyrimidin-4-one

| Conditions | Yield |
|---|---|
| at 150℃; for 24h; | 96.5% |
| at 200℃; for 2h; | 76% |
| at 170℃; for 12h; Neat (no solvent); |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0 - 20℃; for 8h; Inert atmosphere; | 96% |
| In tetrahydrofuran at 0 - 20℃; for 8h; Inert atmosphere; | 96% |
| In diethyl ether for 48h; Ambient temperature; | 82% |
-
-
1603-40-3
3-methylpyridin-2-ylamine

-
-
71-48-7, 917-69-1, 5931-89-5, 55881-15-7, 68931-68-0, 68931-69-1, 93029-27-7
cobalt(II) acetate

-
-
91-15-6
phthalonitrile

-
-
79062-08-1
Co(3-MeBPI)2

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol; toluene 1,2-dicyanobenzene and 2-amino-3-methylpyridine heated in oil bath to 210°C for 3 h, mixt. cooled to ambient temp., toluene added, temp.increased to 90°C, NaOH in methanol added, Co(OAc)2 in methanol added and mixt. heated for 1 h; mixt. cooled to 40°C, product filtered, washed with methanol, diethyl ether and dried; | 96% |
| With sodium hydroxide In methanol; toluene 1,2-dicyanobenzene and 2-amino-3-methylpyridine heated in oil bath to 210°C for 4 h, mixt. cooled to 210°C., toluene added, NaOH in methanol added and stirred for 5 min, Co(OAc)2 in methanol added and mixt. stirredd for 45 min; mixt. cooled to 40°C, product filtered, washed with methanol, diethyl ether and dried in vacuo at 80°C; |


| Conditions | Yield |
|---|---|
| With sulfur In cyclohexane; dimethyl sulfoxide at 120℃; for 1h; Sealed tube; | 96% |
| Multi-step reaction with 2 steps 1: N-iodo-succinimide / dichloromethane / 1 h / 20 °C 2: sodium hydrogencarbonate / dichloromethane / 1.5 h View Scheme |

| Conditions | Yield |
|---|---|
| With fluconazole immobilized chloropropyl-modified Fe3O4-SiO2 core-shell nanoparticle In water at 60℃; for 2h; | 96% |

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; | 96% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-methylpyridin-2-ylamine; phenylglyoxal hydrate In 1,2-dichloro-benzene at 20℃; Sealed tube; Stage #2: hex-1-yne With copper(l) iodide In 1,2-dichloro-benzene at 120℃; for 5h; Sealed tube; regioselective reaction; | 96% |


| Conditions | Yield |
|---|---|
| In water; isopropyl alcohol at 75℃; Microwave irradiation; Green chemistry; | 95% |

| Conditions | Yield |
|---|---|
| cadmium(II) acetate In 1,4-dioxane; water at 160 - 165℃; for 3h; autoclave; | 95% |
2-Amino-3-picoline Chemical Properties
Molecular Structure of 2-Amino-3-methylpyridine(CAS NO.1603-40-3):
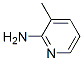
IUPAC Name: 3-methylpyridin-2-amine
Empirical Formula: C6H8N2
Molecular Weight: 108.1411
H bond acceptors: 2
H bond donors: 2
Freely Rotating Bonds: 0
Polar Surface Area: 16.13Å2
Index of Refraction: 1.574
Molar Refractivity: 33.4 cm3
Molar Volume: 101.2 cm3
Surface Tension: 46.9 dyne/cm
Density: 1.068 g/cm3
Flash Point: 109.9 °C
Enthalpy of Vaporization: 45.74 kJ/mol
Boiling Point: 220.9 °C at 760 mmHg
Vapour Pressure: 0.11 mmHg at 25°C
Water Solubility: Soluble
Sensitive: Hygroscopic
BRN: 107892
Melting point: 29-31 °C(lit.)
InChI
InChI=1/C6H8N2/c1-5-3-2-4-8-6(5)7/h2-4H,1H3,(H2,7,8)
Smiles
c1(c(nccc1)N)C
EINECS: 216-501-4
Product Categories: Variousamine; Pyridines, Pyrimidines, Purines and Pteredines; Pyridine series; Pyridine; Pyridines derivates
2-Amino-3-picoline Toxicity Data With Reference
| 1. | orl-rat LD50:100 mg/kg | 85JCAE Prehled Prumyslove Toxikologie; Organicke Latky Marhold, J.,Prague, Czechoslovakia.: Avicenum,1986,841. | ||
| 2. | ihl-rat LCLo:650 ppm/6H | 85JCAE Prehled Prumyslove Toxikologie; Organicke Latky Marhold, J.,Prague, Czechoslovakia.: Avicenum,1986,841. | ||
| 3. | scu-mus LD50:36 mg/kg | AJEBAK Australian Journal of Experimental Biology and Medical Science. 36 (1958),491. | ||
| 4. | ivn-mus LD50:10 mg/kg | CSLNX* U.S. Army Armament Research & Development Command, Chemical Systems Laboratory, NIOSH Exchange Chemicals. (Aberdeen Proving Ground, MD 21010) NX#01585 . | ||
| 5. | skn-gpg LD50:200 mg/kg | 85JCAE Prehled Prumyslove Toxikologie; Organicke Latky Marhold, J.,Prague, Czechoslovakia.: Avicenum,1986,841. |
2-Amino-3-picoline Consensus Reports
Reported in EPA TSCA Inventory.
2-Amino-3-picoline Safety Profile
Poison by ingestion, skin contact, subcutaneous, and intravenous routes. Moderately toxic by inhalation. Combustible. When heated to decomposition it emits toxic fumes of NOx.
Other safty informations about 2-Amino-3-methylpyridine(CAS NO.1603-40-3):
Hazard Codes: T (Toxic)
Risk Statements:
R 23/24/25:Toxic by inhalation, in contact with skin and if swallowed
R33:Take precautionary measures against static discharges
R36/37/38:Irritating to eyes, respiratory system and skin
Safety Statements:
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible)
S28:After contact with skin, wash immediately with plenty of water.
S26 :In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
RIDADR: UN 2811 6.1/PG 3
WGK Germany: 3
2-Amino-3-picoline Specification
2-Amino-3-methylpyridine, with CAS number of 1603-40-3, can be called 2-Amino-3-picoline ; 2-Amino-beta-picoline ; 3-Methyl-2-aminopyridine ; 3-Methyl-2-pyridinamine ; Pyridine, 2-amino-3-methyl- . It is a clear yellow liquid after melting. 2-Amino-3-methylpyridine(CAS NO.1603-40-3) can be used as pharmaceutical raw materials and intermediates.
Related Products
- 2-Amino-3-picoline
- 1603-41-4
- 16034-43-8
- 16034-46-1
- 16034-49-4
- 160348-98-1
- 160357-94-8
- 160369-84-6
- 160369-85-7
- 160369-86-8
- 160377-48-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View