-
Name
2-Aminophenethanol
- EINECS 226-275-9
- CAS No. 5339-85-5
- Article Data19
- CAS DataBase
- Density 1.124 g/cm3
- Solubility soluble in cold water
- Melting Point 89oC
- Formula C8H11NO
- Boiling Point 306.661 °C at 760 mmHg
- Molecular Weight 137.181
- Flash Point 127.913 °C
- Transport Information
- Appearance viscous liquid
- Safety 26-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Phenethylalcohol, o-amino- (6CI,7CI,8CI);2-(2-Aminophenyl)ethanol;2-(2-Aminophenyl)ethyl alcohol;2-(2-Hydroxyethyl)aniline;2-(o-Aminophenyl)ethanol;2-Aminophenethyl alcohol;2-Aminophenylethyl alcohol;NSC 3572;[2-(2-Hydroxyethyl)phenyl]amine;o-Aminophenethyl alcohol;
- PSA 46.25000
- LogP 1.38480
Synthetic route
-
-
15121-84-3
2-nitro-benzeneethanol

-
-
5339-85-5
2-aminophenethyl alcohol

| Conditions | Yield |
|---|---|
| 100% | |
| 100% | |
| 100% |

| Conditions | Yield |
|---|---|
| With hydrogen; palladium/alumina |

| Conditions | Yield |
|---|---|
| With N-benzyl-trimethylammonium hydroxide; calcium chloride; zinc 1.) Me2SO, 95 deg C, 1 h, 2.) H2O, reflux; Multistep reaction; |


| Conditions | Yield |
|---|---|
| With 1-Methylpyrrolidine; selenium In tetrahydrofuran at 150℃; under 22800 Torr; for 5h; | A n/a B 66 % Chromat. |


| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 37 percent / sodium phenoxide / dimethylsulfoxide / 1 h / 60 - 67 °C 2: 78 percent / calcium chloride; zinc powder / H2O / 0.5 h / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: sodium hydroxide / 1.5 h / pH 7.4 / Large scale reaction 2: hydrogen / paraffin oil / 1.5 h / 110 °C / 15001.5 Torr View Scheme | |
| Multi-step reaction with 2 steps 1: sodium phenoxide / dimethyl sulfoxide / 1 h / 60 - 67 °C 2: zinc; calcium chloride / water / 0.5 h / Reflux View Scheme | |
| Multi-step reaction with 2 steps 1: potassium hydroxide / water; dimethyl sulfoxide / 1 h / 0 - 20 °C / Inert atmosphere 2: hydrogen; palladium 10% on activated carbon / methanol / 20.5 h / 20 °C / 760.05 Torr / Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: acetic acid anhydride; nitric acid / 35 - 40 °C / Kochen des Reaktionsprodukts mit methanol. HCl 2: zinc; aqueous calcium chloride View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: borane-THF 2: zinc; calcium chloride; water View Scheme | |
| Multi-step reaction with 2 steps 1: lithium aluminium tetrahydride / methanol 2: 10% Pd/C; hydrogen / ethanol View Scheme |

| Conditions | Yield |
|---|---|
| With tris(2,4-pentanedionato)ruthenium(III); ytterbium(III) trifluoromethanesulfonate nonohydrate; hydrogen; [2-((diphenylphospino)methyl)-2-methyl-1,3-propanediyl]bis[diphenylphosphine] In tetrahydrofuran at 150℃; under 37503.8 Torr; for 15h; Autoclave; | A 61 %Chromat. B n/a |
-
-
30095-98-8
methyl (2-nitrophenyl)acetate

-
A
-
120-72-9
indole

-
B
-
59-48-3
2-oxoindole

-
C
-
5339-85-5
2-aminophenethyl alcohol

-
D
-
35613-44-6
2-aminophenylacetic acid methyl ester

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In tetrahydrofuran; water at 20℃; for 12h; Sonication; chemoselective reaction; |


| Conditions | Yield |
|---|---|
| 100% | |
| 100% | |
| With C21H28I3IrN6Pd; potassium hydroxide In toluene at 110℃; for 2h; Reagent/catalyst; Inert atmosphere; Schlenk technique; | 99% |
-
-
5339-85-5
2-aminophenethyl alcohol

-
-
931105-21-4
2-tert-butyldiphenylsilyloxyethylaniline

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran at 0℃; for 17h; | 100% |
-
-
4509-90-4
5-bromovaleroyl chloride

-
-
5339-85-5
2-aminophenethyl alcohol

-
-
1430091-95-4
5-bromo-N-[2-(2-hydroxyethyl)phenyl]pentanamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at -5 - 0℃; | 100% |
| With triethylamine In dichloromethane at -5 - 0℃; | 100% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 2h; Heating; | 97% |
-
-
994-30-9
triethylsilyl chloride

-
-
5339-85-5
2-aminophenethyl alcohol

-
-
163531-18-8
O-triethylsilyl-2-(o-aminophenyl)ethanol

| Conditions | Yield |
|---|---|
| With triethylamine | 97% |
| With triethylamine In dichloromethane at -20℃; for 1h; | 97% |
-
-
5339-85-5
2-aminophenethyl alcohol

-
-
155164-66-2
2-benzoyl-4,5-dichloropyridazin-3(2H)-one

-
-
364607-96-5
benzoic acid-[2-(2-hydroxy-ethyl)-anilide]

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 18℃; for 18h; | 97% |
-
-
5339-85-5
2-aminophenethyl alcohol

-
-
76-05-1
trifluoroacetic acid

-
-
90732-28-8
2,2,2-trifluoro-1-(indolin-1-yl)ethan-1-one

| Conditions | Yield |
|---|---|
| With tetrachloromethane; triethylamine; triphenylphosphine Heating; | 97% |
-
-
5339-85-5
2-aminophenethyl alcohol

-
-
616-38-6
carbonic acid dimethyl ester

-
-
89875-37-6
2,3-dihydroindole-1-carboxylic acid methyl ester

| Conditions | Yield |
|---|---|
| With 1,3,4,6,7,8-hexahydro-2H-pyrimido[1,2-a]pyrimidine at 90℃; for 21h; Reagent/catalyst; Time; | 97% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-aminophenethyl alcohol With thionyl chloride In 1,2-dimethoxyethane at 20℃; Stage #2: In 1,2-dimethoxyethane; water at 60℃; Further stages.; | 94% |
| With sodium hydroxide; benzenesulfonyl chloride | |
| With zinc(II) chloride at 200℃; |
-
-
5339-85-5
2-aminophenethyl alcohol

-
-
155164-63-9
2-acetyl-4,5-dichloropyridazin-3(2H)-one

-
-
69258-86-2
2-(2-acetylaminophenyl)ethyl alcohol

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 17℃; for 0.2h; | 94% |
-
-
5339-85-5
2-aminophenethyl alcohol

-
-
117917-40-5
2,3-Dioxo-pent-4-enoic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| 94% |

| Conditions | Yield |
|---|---|
| With tetrachloromethane; triethylamine; triphenylphosphine Heating; | 94% |
-
-
10517-21-2
5-chloro-1H-Indole-2-carboxylic acid

-
-
5339-85-5
2-aminophenethyl alcohol

-
-
1036750-32-9
C17H15ClN2O2

| Conditions | Yield |
|---|---|
| With 1-hydroxybenzotriazol-hydrate; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 14h; | 94% |
-
-
5339-85-5
2-aminophenethyl alcohol

-
-
104-87-0
4-methyl-benzaldehyde

-
-
24667-94-5
2-(4-methylphenyl)quinoline

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid; silver trifluoromethanesulfonate In toluene at 120℃; for 12h; Green chemistry; | 94% |

| Conditions | Yield |
|---|---|
| In benzene for 0.5h; Heating; | 93% |
-
-
5339-85-5
2-aminophenethyl alcohol

-
-
155164-68-4
4,5-Dichloro-2-(4-methoxy-benzoyl)-2H-pyridazin-3-one

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 18℃; for 7h; | 93% |
-
-
857070-65-6
3-(3-hydroxy-6-methylpyridin-2-ylmethyl)-2-(3-morpholin-4-ylpropylamino)-3H-benzoimidazole-5-carbaldehyde

-
-
5339-85-5
2-aminophenethyl alcohol

| Conditions | Yield |
|---|---|
| With acetic acid In methanol at 20℃; for 16h; | 93% |
-
-
1101183-58-7
2-isocyanobenzaldehyde

-
-
5339-85-5
2-aminophenethyl alcohol

| Conditions | Yield |
|---|---|
| With pyridinium p-toluenesulfonate In dimethyl sulfoxide at 25℃; Molecular sieve; | 93% |

| Conditions | Yield |
|---|---|
| With [RuCl2(2-diphenylphosphinoethylamine)2]; potassium tert-butylate; zinc(II) trifluoroacetate In 1,4-dioxane for 18h; Reagent/catalyst; Reflux; | 91% |

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 0 - 25℃; | 91% |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
5339-85-5
2-aminophenethyl alcohol

-
-
193806-49-4
2-(2-((tert-butoxycarbonyl)amino)phenyl)ethanol

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In 1,4-dioxane; water for 4h; Ambient temperature; | 90% |
| With sodium hydrogencarbonate In 1,4-dioxane | 72% |
| With sodium hydrogencarbonate In 1,4-dioxane; water at 20℃; |
-
-
29684-56-8
Burgess Reagent

-
-
5339-85-5
2-aminophenethyl alcohol

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 2h; Heating; | 90% |
-
-
5339-85-5
2-aminophenethyl alcohol

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 2h; Heating / reflux; | 90% |

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid; silver trifluoromethanesulfonate In toluene at 120℃; for 12h; Green chemistry; | 89% |
| Multi-step reaction with 2 steps 1: 0.08 h / 100 °C 2: silver trifluoromethanesulfonate; trifluorormethanesulfonic acid; oxygen / toluene / 12 h / 120 °C View Scheme |

| Conditions | Yield |
|---|---|
| With potassium hydroxide | 88% |
| With potassium hydroxide at 180℃; under 0.25 Torr; for 4h; | 78% |
| With potassium hydroxide at 180℃; under 0.05 Torr; for 4h; | 75% |

| Conditions | Yield |
|---|---|
| With sulfuric acid In isopropyl alcohol at 25℃; for 4h; UV-irradiation; | 87% |
-
-
5339-85-5
2-aminophenethyl alcohol

-
-
74-88-4
methyl iodide

-
-
5339-27-5
2-(2-dimethylaminophenyl)ethyl alcohol

| Conditions | Yield |
|---|---|
| Stage #1: 2-aminophenethyl alcohol With potassium carbonate In acetonitrile at 20℃; for 0.666667h; Stage #2: methyl iodide In acetonitrile for 3h; Reflux; | 86.3% |

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid; silver trifluoromethanesulfonate In toluene at 120℃; for 12h; Green chemistry; | 84% |

| Conditions | Yield |
|---|---|
| With tetrachloromethane; triethylamine; triphenylphosphine Heating; | 83% |
2-Aminophenethanol Specification
The 2-Aminophenethanol, with the CAS registry number 5339-85-5, is also known as o-Aminophenethyl alcohol. It belongs to the product categories of Naphthyridine,Quinoline; Amino Alcohols; Organic Building Blocks; Oxygen Compounds. Its EINECS number is 226-275-9. This chemical's molecular formula is C8H11NO and molecular weight is 137.18. What's more, its systematic name is 2-(2-Aminophenyl)ethanol. This chemical should be sealed and stored in a dry place. Moreover, it should be protected from light and oxidants. It is used in organic synthesis.
Physical properties of 2-Aminophenethanol are: (1)ACD/LogP: 0.17; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0.14; (4)ACD/LogD (pH 7.4): 0.17; (5)ACD/BCF (pH 5.5): 1.00; (6)ACD/BCF (pH 7.4): 1.00; (7)ACD/KOC (pH 5.5): 27.67; (8)ACD/KOC (pH 7.4): 29.45; (9)#H bond acceptors: 2; (10)#H bond donors: 3; (11)#Freely Rotating Bonds: 4; (12)Polar Surface Area: 46.25 Å2; (13)Index of Refraction: 1.597; (14)Molar Refractivity: 41.573 cm3; (15)Molar Volume: 122.037 cm3; (16)Polarizability: 16.481×10-24cm3; (17)Surface Tension: 51.19 dyne/cm; (18)Density: 1.124 g/cm3; (19)Flash Point: 127.913 °C; (20)Enthalpy of Vaporization: 57.778 kJ/mol; (21)Boiling Point: 306.661 °C at 760 mmHg; (22)Vapour Pressure: 0 mmHg at 25°C.
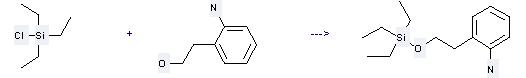
Uses of 2-Aminophenethanol: it can be used to produce 2-(2-triethylsilanyloxy-ethyl)-phenylamine at the temperature of -20 °C. It will need reagent Et3N and solvent CH2Cl2 with the reaction time of 1 hour. The yield is about 97%.
When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to eyes, respiratory system and skin. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. When using it, you need to wear suitable protective gloves and eye/face protection.
You can still convert the following datas into molecular structure:
(1)SMILES: OCCc1ccccc1N
(2)Std. InChI: InChI=1S/C8H11NO/c9-8-4-2-1-3-7(8)5-6-10/h1-4,10H,5-6,9H2
(3)Std. InChIKey: ILDXSRFKXABMHH-UHFFFAOYSA-N
Related Products
- 2-Aminophenethanol
- 53398-55-3
- 53398-80-4
- 53398-83-7
- 53398-85-9
- 53398-86-0
- 53399-81-8
- 53400-41-2
- 5340-04-5
- 534-00-9
- 534-03-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View