-
Name
2-Aminopurine
- EINECS 207-197-4
- CAS No. 452-06-2
- Article Data23
- CAS DataBase
- Density 1.612 g/cm3
- Solubility Soluble in acid, slightly soluble in hot ethanol, insoluble in alkaline solution
- Melting Point 280-282 °C(lit.)
- Formula C5H5N5
- Boiling Point 616.3 °C at 760 mmHg
- Molecular Weight 135.128
- Flash Point 362.9 °C
- Transport Information
- Appearance White to light yellow crystal powder
- Safety 22-36-26
- Risk Codes 22-36/37/38-20/21/22
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms 1H-Purin-2-amine(9CI);Purine, 2-amino- (6Cl,8Cl);2-Aminopurine;Isoadenine;NSC 24129;SQ22451;CCRIS 759;Purine, 2-amino-;
- PSA 80.48000
- LogP 0.51630
Synthetic route
-
-
33512-51-5
2-(methylthio)-9H-purine

-
-
452-06-2
9H-purin-2-amine

| Conditions | Yield |
|---|---|
| With potassium amide In ammonia for 70h; Mechanism; 2-halogenated purines; | 90% |
-
-
10310-21-1
2-Amino-6-chloropurin

-
-
452-06-2
9H-purin-2-amine

| Conditions | Yield |
|---|---|
| In hydrogenchloride at 50℃; electrolysis, -0.75V, initial current 50-60 mA; | 85% |
| palladium on charcoal In sodium hydroxide; water |

-
-
452-06-2
9H-purin-2-amine

| Conditions | Yield |
|---|---|
| With methylamine In methanol at 100℃; for 17h; | 80% |

| Conditions | Yield |
|---|---|
| With ammonia In methanol at 100℃; for 17h; Mechanism; Product distribution; | A 70% B 20% |

| Conditions | Yield |
|---|---|
| 1.) RT, 6 h, 2.) reflux, 30 min; | 61% |

-
A
-
33512-51-5
2-(methylthio)-9H-purine

-
B
-
452-06-2
9H-purin-2-amine

| Conditions | Yield |
|---|---|
| With ammonia In methanol at 100℃; for 17h; Product distribution; Mechanism; ANRORC and no ANRORC mechanism determined by reaction with 15N labelled ammonia; var. temp. and time; | A 55% B 25% |
-
-
61991-08-0
amipurimycin

-
-
452-06-2
9H-purin-2-amine

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid at 130℃; | 48% |

-
-
452-06-2
9H-purin-2-amine

| Conditions | Yield |
|---|---|
| With nickel In water for 7.5h; Heating; | 35.7% |
-
-
107550-74-3
iso-2',3'-dideoxyadenosine

-
A
-
122999-44-4
2,3-Didesoxy-β-D-glycero-pentofuranose

-
B
-
452-06-2
9H-purin-2-amine

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 22℃; rate of hydrolysis relative to dideoxyadenosine; |
-
-
3616-24-8
2-amino-9-(β-D-2'-deoxyribofuranosyl)-purine

-
A
-
18620-60-5
2,4-diamino-5-formamidopyrimidine

-
B
-
452-06-2
9H-purin-2-amine

| Conditions | Yield |
|---|---|
| With sodium cacodylate buffer; water at 110℃; for 22h; Kinetics; Thermodynamic data; Mechanism; ΔH(excit.); var. temp. and times; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 60 percent / NaOH / dioxane; H2O / 5 - 10 °C 2: 35.7 percent / Raney nickel / H2O / 7.5 h / Heating View Scheme |
-
-
77287-34-4, 77287-35-5, 60100-09-6
formamide

-
-
452-06-2
9H-purin-2-amine

| Conditions | Yield |
|---|---|
| With Canyon Diablo iron meteorites at 140℃; for 24h; Reagent/catalyst; Temperature; |
-
-
452-06-2
9H-purin-2-amine

| Conditions | Yield |
|---|---|
| With thallium (I) ethoxide In ethanol at 15 - 20℃; for 36h; | 92% |
-
-
452-06-2
9H-purin-2-amine

-
-
56287-17-3, 69123-94-0, 784-71-4
2'-deoxy-2'-fluorouridine

| Conditions | Yield |
|---|---|
| In water at 37℃; for 48h; thymidine phosphorylase, purine nucleoside phosphorylase, phosphate buffer, pH 7; | 89% |
-
-
452-06-2
9H-purin-2-amine

-
-
951-77-9
2'-Deoxycytidine

-
-
3616-24-8
2-amino-9-(β-D-2'-deoxyribofuranosyl)-purine

| Conditions | Yield |
|---|---|
| With citrate buffer; N-deoxyribosyltransferase from L. leichmannii In ethanol at 40℃; for 48h; | 86% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 50 - 80℃; | 85% |
-
-
452-06-2
9H-purin-2-amine

-
-
157363-84-3
1,2:5,6-dianhydro-3,4-di-O-benzyl-L-iditol

| Conditions | Yield |
|---|---|
| Stage #1: 9H-purin-2-amine With caesium carbonate In N,N-dimethyl-formamide at 20℃; for 2h; Stage #2: 1,2:5,6-dianhydro-3,4-di-O-benzyl-L-iditol In N,N-dimethyl-formamide at 110℃; | 66% |

| Conditions | Yield |
|---|---|
| at 130℃; for 17h; | 63% |
-
-
36016-40-7
mesitylenesulfonylhydroxylamine

-
-
452-06-2
9H-purin-2-amine

| Conditions | Yield |
|---|---|
| In methanol; dichloromethane for 4h; Ambient temperature; | 62% |

| Conditions | Yield |
|---|---|
| With phosphate buffer; cell paste of Enterobacter aerogenes AJ 11125 at 60℃; for 15h; pH 7.0; | 61% |
-
-
127047-77-2
4-acetoxy-3-(acetoxymethyl)-1-iodobutane

-
-
452-06-2
9H-purin-2-amine

-
A
-
131266-15-4
7-(4-acetoxy-3-acetoxymethylbutyl)-2-aminopurine

-
B
-
104227-87-4
9-(4-acetoxy-3-acetoxymethylbutyl)-2-aminopurine

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide for 18h; Ambient temperature; | A 15% B 59% |

-
-
13831-03-3
tert-butyl prop-2-ynoate

-
-
452-06-2
9H-purin-2-amine

-
A
-
1043904-89-7
C12H15N5O2

-
B
-
1043904-90-0
C12H15N5O2

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 50 - 80℃; | A 57% B 23% |

-
-
452-06-2
9H-purin-2-amine

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 20 - 65℃; for 8h; | A 20% B 53% |

-
-
105-36-2
ethyl bromoacetate

-
-
452-06-2
9H-purin-2-amine

-
-
933477-63-5
2-(2-amino-9H-purin-9-yl)acetic acid

| Conditions | Yield |
|---|---|
| Stage #1: 9H-purin-2-amine With sodium hydride In N,N-dimethyl-formamide at 0 - 20℃; for 1h; Inert atmosphere; Stage #2: ethyl bromoacetate In N,N-dimethyl-formamide for 2h; Inert atmosphere; Stage #3: With sodium hydroxide In water; N,N-dimethyl-formamide at 20℃; for 1h; Inert atmosphere; | 51% |
-
-
1134003-26-1
(5aS,7S)-7-[(1R/S)-2-bromo-1-methylethyl]-3-methyl-1H,7H-5a,6,8,9-tetrahydro-1-oxopyrano[4,3-b][1]benzopyran

-
-
452-06-2
9H-purin-2-amine

-
-
1134003-03-4
(5aS,7S)-7-[(1R/S)-2-(2-amino-9H-purin-9-yl)-1-methylethyl]-3-methyl-1H,7H-5a,6,8,9-tetrahydro-1-oxopyrano[4,3-b][1]benzopyran

| Conditions | Yield |
|---|---|
| Stage #1: 9H-purin-2-amine With caesium carbonate In N,N-dimethyl-formamide at 25℃; for 1h; Inert atmosphere; Stage #2: (5aS,7S)-7-[(1R/S)-2-bromo-1-methylethyl]-3-methyl-1H,7H-5a,6,8,9-tetrahydro-1-oxopyrano[4,3-b][1]benzopyran In N,N-dimethyl-formamide at 25℃; for 24h; Inert atmosphere; | 48% |
-
-
4362-40-7
5-chloromethyl-2,2-dimethyl-1,3-dioxolane

-
-
452-06-2
9H-purin-2-amine

-
A
-
120139-11-9
9-(RS)-(2,2-dimethyl-1,3-dioxolan-4-yl)methyl-2-aminopurine

-
B
-
120139-12-0
7-(RS)-(2,2-dimethyl-1,3-dioxolan-4-yl)methyl-2-aminopurine

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 100℃; for 14h; | A 44% B 15% |


| Conditions | Yield |
|---|---|
| at 50℃; for 2h; Escherichia coli JA-300 cells, pH 6.5; | 44% |
-
-
116384-57-7
Diethyl 2-Bromoethoxymethanephosphonate

-
-
452-06-2
9H-purin-2-amine

-
-
126354-35-6
[2-(2-Amino-purin-9-yl)-ethoxymethyl]-phosphonic acid diethyl ester

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide a) At 80 deg C for 1 h 2) addition of IIc,80 deg C for 16 h; | 40% |
-
-
51385-79-6
diethyl-2-(2-bromoethylidene)malonte

-
-
452-06-2
9H-purin-2-amine

-
-
134470-59-0
2-amino-9-(2,2-dicarboethoxycyclopropyl)purine

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide | 39% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 70℃; for 4h; | 36% |
-
-
452-06-2
9H-purin-2-amine

-
-
850883-62-4
2-deoxy-2-fluoro-α-D-arabinofuranose 1-phosphate

| Conditions | Yield |
|---|---|
| purine nucleoside phosphorylase In water at 50℃; for 216h; pH=7.5; Aqueous phosphate buffer; Enzymatic reaction; | 32% |

| Conditions | Yield |
|---|---|
| With ethanol 1) EtOH, 25 min, reflux, 2) 15 min, reflux; Yield given. Multistep reaction; |
2-Aminopurine Specification
The 2-Aminopurine with CAS registry number of 452-06-2 is also known as Purine, 2-amino- (6Cl,8Cl). The IUPAC name is 7H-Purin-2-amine. It belongs to product categories of Pyrimidine; Purine; Nucleotides and Nucleosides; Purines; Biochemistry; Nucleobases and Their Analogs; Nucleosides, Nucleotides & Related Reagents; Nucleic Acids; Bases & Related Reagents; Nucleotides; Nucleoside Analogs; Nucleosides, Nucleotides, Oligonucleotides; Biochemicals and Reagents. Its EINECS registry number is 207-197-4. In addition, the formula is C5H5N5 and the molecular weight is 135.15.
Physical properties about 2-Aminopurine are: (1)ACD/LogP: -0.52; (2)ACD/LogD (pH 5.5): -0.57; (3)ACD/LogD (pH 7.4): -0.53; (4)ACD/BCF (pH 5.5): 1; (5)ACD/BCF (pH 7.4): 1; (6)ACD/KOC (pH 5.5): 11.11; (7)ACD/KOC (pH 7.4): 12.31; (8)#H bond acceptors: 5; (9)#H bond donors: 3; (10)#Freely Rotating Bonds: 0; (11)Index of Refraction: 1.837; (12)Molar Refractivity: 37.03 cm3; (13)Molar Volume: 83.8 cm3; (14)Surface Tension: 122.7 dyne/cm; (15)Density: 1.612 g/cm3; (16)Flash Point: 362.9 °C; (17)Enthalpy of Vaporization: 91.41 kJ/mol; (18)Boiling Point: 616.3 °C at 760 mmHg; (19)Vapour Pressure: 4.03E-15 mmHg at 25 °C.
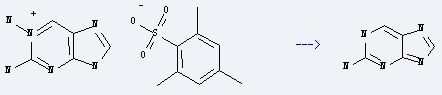
Preparation of 2-Aminopurine: it is prepared by reaction of 1,2-diaminopurinium mesitylenesulphonate. The reaction needs reagent methylamine and solvent methanol at the temperature of 100 °C for 17 hours. The yield is about 80%. It is a fluorescent molecular marker used in nucleic acid research and sometimes used in the laboratory for mutagenesis.
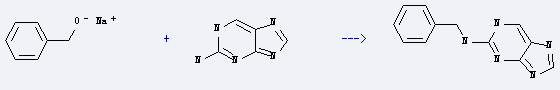
Uses of 2-Aminopurine: it is used to produce 2-benzylaminopurine by reaction with phenylmethanol; sodium salt. The reaction occurs at the temperature of 130 °C for 17 hours. The yield is about 63%.
When you are using this chemical, please be cautious about it. As a chemical, it is irritating to eyes, respiratory system and skin. What's more, it is harmful by inhalation, in contact with skin and if swallowed. During using it, wear suitable protective clothing and do not breathe dust. If contact with eyes accidently, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: C1=C2C(=NC(=N1)N)N=CN2
2. InChI: InChI=1S/C5H5N5/c6-5-7-1-3-4(10-5)9-2-8-3/h1-2H,(H3,6,7,8,9,10)
3. InChIKey: MWBWWFOAEOYUST-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | intraperitoneal | 270mg/kg (270mg/kg) | Japanese Kokai Tokyo Koho Patents. Vol. #78-55733, | |
| rat | LD50 | oral | 723mg/kg (723mg/kg) | Japanese Kokai Tokyo Koho Patents. Vol. #78-55733, |
Related Products
- 2-Aminopurine
- 2-Aminopurine riboside
- 452-07-3
- 452077-13-3
- 452-08-4
- 452-09-5
- 452105-23-6
- 452-10-8
- 452-11-9
- 4521-22-6
- 4521-28-2
- 4521-30-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View