-
Name
2-Bromo-2',4'-dichloroacetophenone
- EINECS 220-116-7
- CAS No. 2631-72-3
- Article Data60
- CAS DataBase
- Density 1.695 g/cm3
- Solubility
- Melting Point 25-29 ºC
- Formula C8H5 Br Cl2 O
- Boiling Point 296.7 °C at 760 mmHg
- Molecular Weight 267.937
- Flash Point 133.3 °C
- Transport Information
- Appearance White to brown low melting solid
- Safety S26;S36 25 28A 36 37 39 45
- Risk Codes R36/37/38
-
Molecular Structure
-
Hazard Symbols

- Synonyms Acetophenone,2-bromo-2',4'-dichloro- (7CI); 2,4-Dichlorophenacyl bromide; 2,4-Dichlorophenylbromomethyl ketone; 2-Bromo-1-(2,4-dichlorophenyl)ethan-1-one;2-Bromo-1-(2,4-dichlorophenyl)ethanone; 2-Bromo-2',4'-dichloroacetophenone;2',4'-Dichloro-2-bromoacetophenone; a-Bromo-2,4-dichloroacetophenone; w-Bromo-2,4-dichloroacetophenone
- PSA 17.07000
- LogP 3.57100
Synthetic route

| Conditions | Yield |
|---|---|
| With bromine In 1,4-dioxane; diethyl ether for 0.5h; Ambient temperature; | 100% |
| With copper(ll) bromide In chloroform; ethyl acetate at 40℃; for 2h; | 100% |
| With dihydrogen peroxide; bromine; sodium carbonate In dichloromethane at 20 - 40℃; Green chemistry; | 91.4% |
-
-
2234-16-4
1-(2,4-dichlorophenyl)ethan-1-one

-
A
-
24123-68-0
2,2-dibromo-1-(2,4-dichlorophenyl)ethanone

-
B
-
2631-72-3
2,4-dichlorophenacyl bromide

| Conditions | Yield |
|---|---|
| With trimethylsilyl bromide; potassium nitrate In dichloromethane at 20℃; for 48h; | A n/a B 72% |
-
-
24123-68-0
2,2-dibromo-1-(2,4-dichlorophenyl)ethanone

-
-
121-45-9
phosphorous acid trimethyl ester

-
A
-
2631-72-3
2,4-dichlorophenacyl bromide

-
B
-
68107-01-7
O,O-dimethyl-O-<1-(2,4-dichlorophenyl)-2-bromo>vinyl phosphate (isomer Z)

| Conditions | Yield |
|---|---|
| In methanol for 0.25h; Heating; Title compound not separated from byproducts; | A 45% B 10% |

-
-
2234-16-4
1-(2,4-dichlorophenyl)ethan-1-one

-
A
-
60208-07-3
α-bromo-2,4-dibromoacetophenone

-
B
-
2631-72-3
2,4-dichlorophenacyl bromide

| Conditions | Yield |
|---|---|
| With bromine In acetic acid at 35 - 40℃; for 2h; |
-
-
60-29-7
diethyl ether

-
-
7732-18-5
water

-
-
2234-16-4
1-(2,4-dichlorophenyl)ethan-1-one

-
-
2631-72-3
2,4-dichlorophenacyl bromide

| Conditions | Yield |
|---|---|
| With bromine |

-
-
13094-51-4
bromoacetic anhydride

-
-
541-73-1
1,3-Dichlorobenzene

-
-
2631-72-3
2,4-dichlorophenacyl bromide

| Conditions | Yield |
|---|---|
| Stage #1: bromoacetic anhydride With aluminum (III) chloride In dichloromethane at 25℃; for 0.5h; Stage #2: 1,3-Dichlorobenzene In dichloromethane for 16h; Reflux; |

| Conditions | Yield |
|---|---|
| Stage #1: 2-Bromoacetyl bromide With aluminum (III) chloride In dichloromethane at 25℃; for 0.5h; Stage #2: 1,3-Dichlorobenzene In dichloromethane for 16h; Reflux; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: aluminum (III) chloride / 1,2-dichloro-benzene / 0.33 h 1.2: Cooling with ice 2.1: aluminum (III) chloride; bromine / diethyl ether / Cooling with ice View Scheme | |
| Multi-step reaction with 2 steps 1: calcium chloride; hydrogenchloride; aluminum (III) chloride / water / 0.83 h 2: aluminum (III) chloride; bromine / diethyl ether / Cooling with ice View Scheme | |
| Multi-step reaction with 2 steps 1: 4 h / 60 °C / Ionic liquid; Green chemistry 2: dihydrogen peroxide; sodium carbonate; bromine / dichloromethane / 20 - 40 °C / Green chemistry View Scheme |
-
-
22118-09-8
2-bromoacetyl chloride

-
-
541-73-1
1,3-Dichlorobenzene

-
-
2631-72-3
2,4-dichlorophenacyl bromide

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride for 0.666667h; Friedel-Crafts Acylation; Cooling with ice; Inert atmosphere; |
-
-
2631-72-3
2,4-dichlorophenacyl bromide

-
-
63698-52-2
2-mercapto-5-(3,4-methylenedioxyphenyl)-1,3,4-oxadiazole

-
-
146942-90-7
2-(5-Benzo[1,3]dioxol-5-yl-[1,3,4]oxadiazol-2-ylsulfanyl)-1-(2,4-dichloro-phenyl)-ethanone

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol | 98% |
-
-
2631-72-3
2,4-dichlorophenacyl bromide

-
-
187164-20-1
(S)-2-bromo-1-(2,4-dichlorophenyl)ethan-1-ol

| Conditions | Yield |
|---|---|
| With B-chlorodiisopinocampheylborane In tetrahydrofuran at -25℃; for 16h; | 98% |
| Stage #1: 2,4-dichlorophenacyl bromide With borane Ν,Ν-diethylaniline complex; (S)-1-methyl-3,3-diphenyl-hexahydropyrrolo[1,2-c][1,3,2]oxazaborole In tetrahydrofuran; toluene at 20 - 30℃; for 1h; Stage #2: With methanol In tetrahydrofuran; toluene Cooling; | 72% |
-
-
2631-72-3
2,4-dichlorophenacyl bromide

-
-
95-54-5
1,2-diamino-benzene

-
-
930781-40-1
2-(2, 4-dichlorophenyl)quinoxaline

| Conditions | Yield |
|---|---|
| With polymeric resin-bound hexafluorophosphate ion In methanol; water at 20℃; for 6.5h; | 98% |
| With potassium fluoride on basic alumina at 20℃; for 2h; | 93% |
| With γ-maghemite-silica nanocomposite In neat (no solvent) for 6h; Green chemistry; | 88% |
-
-
2631-72-3
2,4-dichlorophenacyl bromide

-
-
109-77-3
malononitrile

-
-
141776-18-3
2-(2-(3,4-dichlorophenyl)-2-oxoethyl)malononitrile

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran | 97.5% |
-
-
2631-72-3
2,4-dichlorophenacyl bromide

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 0 - 20℃; for 1.8h; | 97% |

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol for 4h; Reflux; | 97% |


| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In benzene for 30h; Reflux; | 96% |
| With toluene-4-sulfonic acid In butan-1-ol; benzene for 6h; Reflux; | 92.54% |
| With toluene-4-sulfonic acid In butan-1-ol; benzene at 87 - 88.5℃; for 5h; Reagent/catalyst; Solvent; Temperature; | 83.22% |
-
-
79-19-6
thiosemicarbazide

-
-
2631-72-3
2,4-dichlorophenacyl bromide

-
-
529-34-0
3,4-dihydronaphthalene-1(2H)-one

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; for 0.0833333h; Solvent; Temperature; regioselective reaction; | 96% |


| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide In ethyl acetate at 90℃; for 3h; | 96% |
-
-
2631-72-3
2,4-dichlorophenacyl bromide

-
-
23269-92-3
5-(3,4,5-trimethoxyphenyl)-1,3,4-oxadiazole-2-thiol

-
-
146942-88-3
1-(2,4-Dichloro-phenyl)-2-[5-(3,4,5-trimethoxy-phenyl)-[1,3,4]oxadiazol-2-ylsulfanyl]-ethanone

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol | 95% |
| In acetone at 40℃; for 1h; | 80% |
-
-
2631-72-3
2,4-dichlorophenacyl bromide

-
-
37687-24-4
diethyl 1H-pyrazole-3,5-dicarboxylate

-
-
1101323-82-3
diethyl 1-[2-(2,4-dichlorophenyl)-2-oxoethyl]-1H-pyrazole-3,5-dicarboxylate

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 20℃; for 12h; | 94% |
-
-
2631-72-3
2,4-dichlorophenacyl bromide

-
-
53066-15-2
2-bromo-1-(2,4-dichlorophenyl)ethanol

| Conditions | Yield |
|---|---|
| Stage #1: 2,4-dichlorophenacyl bromide With sodium tetrahydroborate In methanol at 0 - 20℃; for 1.5h; Inert atmosphere; Stage #2: With water In methanol | 94% |
| With sodium tetrahydroborate In isopropyl alcohol at 3 - 20℃; for 2h; | 57% |
-
-
418776-10-0
5-{[4-(2-methoxyphenyl)piperazin-1-yl]methyl}-4-phenyl-4H-1,2,4-triazole-3-thiol

-
-
2631-72-3
2,4-dichlorophenacyl bromide

| Conditions | Yield |
|---|---|
| Stage #1: 5-{[4-(2-methoxyphenyl)piperazin-1-yl]methyl}-4-phenyl-4H-1,2,4-triazole-3-thiol With sodium ethanolate In ethanol for 0.0666667h; Sonication; Green chemistry; Stage #2: 2,4-dichlorophenacyl bromide In ethanol for 0.1h; Sonication; Green chemistry; | 94% |

-
-
2631-72-3
2,4-dichlorophenacyl bromide

| Conditions | Yield |
|---|---|
| Stage #1: 5-{[4-(2-methoxyphenyl)piperazin-1-yl]methyl}-4-benzyl-4H-1,2,4-triazole-3-thiol With sodium ethanolate In ethanol for 0.0666667h; Sonication; Green chemistry; Stage #2: 2,4-dichlorophenacyl bromide In ethanol for 0.1h; Sonication; Green chemistry; | 94% |

| Conditions | Yield |
|---|---|
| With triethylamine In acetone at 55℃; for 0.333333h; Sonication; Green chemistry; | 94% |

| Conditions | Yield |
|---|---|
| In ethanol; N,N-dimethyl-formamide at 80℃; for 5h; | 93% |

| Conditions | Yield |
|---|---|
| Stage #1: thiosemicarbazide; 3-Nitroacetophenone With acetic acid at 70 - 75℃; for 1.3h; Green chemistry; Stage #2: 2,4-dichlorophenacyl bromide at 70 - 75℃; for 0.416667h; Green chemistry; | 93% |

-
-
74227-66-0
1-indanone thiosemicarbazone

-
-
2631-72-3
2,4-dichlorophenacyl bromide

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol at 80℃; | 93% |
-
-
288-32-4
1H-imidazole

-
-
2631-72-3
2,4-dichlorophenacyl bromide

-
-
46503-52-0
1-(2,4-dichlorophenyl)-2-(imidazol-1-yl)ethanone

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 10℃; Reagent/catalyst; | 92% |
| In tetrahydrofuran at 20℃; for 1.5h; Inert atmosphere; | 87% |
| With potassium carbonate at 20℃; | 78% |

-
-
2631-72-3
2,4-dichlorophenacyl bromide

| Conditions | Yield |
|---|---|
| In ethanol at 100℃; under 7500.75 Torr; for 0.0833333h; Hantzsch Thiazole Synthesis; Microwave irradiation; | 92% |
-
-
14063-77-5, 88438-06-6
(Z)-3-chloro-3-(4-chlorophenyl)acrylaldehyde

-
-
79-19-6
thiosemicarbazide

-
-
2631-72-3
2,4-dichlorophenacyl bromide

| Conditions | Yield |
|---|---|
| Stage #1: (Z)-3-chloro-3-(4-chlorophenyl)acrylaldehyde; thiosemicarbazide With acetic acid at 70 - 75℃; Green chemistry; Stage #2: 2,4-dichlorophenacyl bromide Heating; Green chemistry; | 92% |


| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene for 4h; Reflux; Green chemistry; | 91.9% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene for 4h; Reflux; | 91.65% |

-
-
2631-72-3
2,4-dichlorophenacyl bromide

| Conditions | Yield |
|---|---|
| Stage #1: C22H25FN6O2S With sodium ethanolate In ethanol for 0.0666667h; Sonication; Green chemistry; Stage #2: 2,4-dichlorophenacyl bromide In ethanol for 0.1h; Sonication; Green chemistry; | 91% |
-
-
2631-72-3
2,4-dichlorophenacyl bromide

-
-
63698-51-1
5-(4-ethoxy-3,5-dimethoxy-phenyl)-3H-[1,3,4]oxadiazole-2-thione

-
-
146942-94-1
1-(2,4-Dichloro-phenyl)-2-[5-(4-ethoxy-3,5-dimethoxy-phenyl)-[1,3,4]oxadiazol-2-ylsulfanyl]-ethanone

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol | 90% |
-
-
2631-72-3
2,4-dichlorophenacyl bromide

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In ethanol 15-20 min, reflux, 1-2 h, r.t.; | 90% |
-
-
2631-72-3
2,4-dichlorophenacyl bromide

-
-
121-45-9
phosphorous acid trimethyl ester

-
A
-
68107-01-7
O,O-dimethyl-O-<1-(2,4-dichlorophenyl)-2-bromo>vinyl phosphate (isomer Z)

-
C
-
2234-16-4
1-(2,4-dichlorophenyl)ethan-1-one

| Conditions | Yield |
|---|---|
| In methanol for 0.25h; Title compound not separated from byproducts; | A 90% B 3.3% C 3.3% |
| In methanol for 0.25h; Mechanism; Heating; other phenacyl and phenacylidene halides; |

-
-
219474-93-8
4-amino-3-(D-glucoheptonic-hexitol-1-yl)-1H-1,2,4-triazole-5-thione

-
-
2631-72-3
2,4-dichlorophenacyl bromide

-
-
1027801-77-9
6-(2,4-dichlorophenyl)-3-(D-glucoheptonic-hexitol-1-yl)-7H-1,2,4-triazolo[3,4-b][1,3,4]thiadiazine

| Conditions | Yield |
|---|---|
| In ethanol for 4h; Heating; | 90% |
-
-
30748-47-1
5-acetyl-4-methylthiazole-2-amine

-
-
2631-72-3
2,4-dichlorophenacyl bromide

| Conditions | Yield |
|---|---|
| With polyethylene glycol (PEG-400) In water for 0.133333h; Microwave irradiation; Green chemistry; | 90% |
2-Bromo-2',4'-dichloroacetophenone Chemical Properties
Molecular Structure of 2-Bromo-2',4'-dichloroacetophenone (CAS NO.2631-72-3):
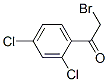
IUPAC: 2-Bromo-1-(2,4-dichlorophenyl)ethanone
Molecular Formula: C8H5BrCl2O
Molecular Weight: 267.93
EINECS: 220-116-7
Product Categories: C7 to C8;Carbonyl Compounds;Ketones;Acetophenone series
Melting Point: 25-29oC(lit.)
Density: 1.695 g/cm3
Flash Point: 133.3oC
Boiling Point: 296.7oC at 760 mmHg
Sensitive: Lachrymatory
Index of Refraction: 1.596
Molar Volume: 158 cm3
Surface Tension: 48.1 dyne/cm
Molar Refractivity: 53.8 cm3
Enthalpy of Vaporization: 53.65 kJ/mol
Vapour Pressure: 0.00141 mmHg at 25oC
SMILES: O=C(c1ccc(Cl)cc1Cl)CBr
InChI: InChI=1/C8H5BrCl2O/c9-4-8(12)6-2-1-5(10)3-7(6)11/h1-3H,4H2
InChIKey: DASJDMQCPIDJIF-UHFFFAOYAW
2-Bromo-2',4'-dichloroacetophenone Safety Profile
Safety Information of 2-Bromo-2',4'-dichloroacetophenone (CAS NO.2631-72-3):
Hazard Codes: Xi ,C
,C
Risk Statements: 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 26-36
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36:Wear suitable protective clothing.
WGK Germany: 3
Hazard Note: Corrosive/Lachrymatory
2-Bromo-2',4'-dichloroacetophenone Specification
2-Bromo-2',4'-dichloroacetophenone with cas registry number of 2631-72-3 is also known as 2-Brom-1-(2,4-dichlorphenyl)ethanon ; 2-Bromo-1-(2,4-dichlorophenyl)ethanone ; Ethanone, 2-bromo-1-(2,4-dichlorophenyl)- ; 2,4-Dichlorophenacyl bromide ; 2-Bromo-1-(2,4-dichlorophenyl)ethan-1-one ; 2-Bromo-2',4'-dichloroacetophenone . It is a carbonyl compound with the appearance of white to brown low melting solid . It should be stored below 4oC in a dry,corrosives area and stored under argon . The raw materials are Chloroform , Aluminium chloride , 1,3-Dichlorobenzene . 2-Bromo-2',4'-dichloroacetophenone with cas registry number of 2631-72-3 is used as a pharmaceutical intermediate .
Related Products
- 2-Bromo-2-(2'-chlorophenyl) acetic acid
- 2-Bromo-2-(2-fluorophenyl)-1-cyclopropylethanone
- 2-Bromo-2-(diethoxyphosphoryl)acetic acid
- 2-Bromo-2,2-dichloroacetic acid ethyl ester
- 2-Bromo-2,2-difluoroacetamide
- 2-Bromo-2,2-difluoroethylamine
- 2-Bromo-2',4'-dichloroacetophenone
- 2-Bromo-2',4'-difluoroacetophenone
- 2-Bromo-2',5'-dimethoxyacetophenone
- 2-Bromo-2',6'-dichloroacetophenone
- 26317-70-4
- 2631-77-8
- 2632-10-2
- 2632-13-5
- 26322-14-5
- 26322-20-3
- 26323-02-4
- 26323-88-6
- 263244-95-7
- 26326-05-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View