-
Name
2-Bromo-2-(2-fluorophenyl)-1-cyclopropylethanone
- EINECS 812-641-0
- CAS No. 204205-33-4
- Article Data21
- CAS DataBase
- Density 1.574 g/cm3
- Solubility
- Melting Point
- Formula C11H10BrFO
- Boiling Point 293.012 °C at 760 mmHg
- Molecular Weight 257.102
- Flash Point 131.009 °C
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 2-Bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone;Ethanone,2-bromo-1-cyclopropyl-2-(2-fluorophenyl)-;a-(Cyclopropylcarbonyl)-2-fluorobenzyl bromide;
- PSA 17.07000
- LogP 3.24080
Synthetic route
-
-
150322-73-9
1-cyclopropyl-2-(2-fluorophenyl)ethanone

-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone

| Conditions | Yield |
|---|---|
| With iodine(I) bromide; 1-n-butyl-3-methylimidazolim bromide In tetrahydrofuran at 40℃; for 4h; Temperature; Time; Concentration; | 95.3% |
| With bromine In methanol at 25 - 30℃; for 6h; Concentration; | 94.65% |
| With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione; 2,2'-azobis(isobutyronitrile) In cyclohexane at 10℃; Product distribution / selectivity; Reflux; | 93.28% |
-
-
451-82-1
(2-fluorophenyl)acetic acid

-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: triethylamine / dichloromethane / 0.25 h / 0 - 5 °C 1.2: 6 h / 0 - 5 °C 2.1: iodine; magnesium / tetrahydrofuran / 25 - 50 °C 2.2: 40 - 50 °C 2.3: 0.25 h / 0 - 30 °C 3.1: toluene-4-sulfonic acid; 2,2'-azobis(isobutyronitrile); N-Bromosuccinimide / chloroform / 4 h / Reflux View Scheme | |
| Multi-step reaction with 2 steps 1.1: magnesium; isopropyl bromide / toluene; tetrahydrofuran / 15 - 65 °C 1.2: 5 - 65 °C 2.1: dibenzoyl peroxide; N-Bromosuccinimide / ethyl acetate / 25 - 60 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: magnesium; isopropyl bromide / toluene; tetrahydrofuran / 15 - 65 °C 1.2: 5 - 65 °C 2.1: dibenzoyl peroxide; N-Bromosuccinimide / ethyl acetate / 25 - 60 °C View Scheme | |
| Multi-step reaction with 2 steps 1: sodium hydride / toluene; dimethyl sulfoxide / 1.5 h / 95 - 110 °C / Large scale 2: bromine / methanol / Large scale View Scheme |
-
-
946402-23-9
2-(2-fluorophenyl)-N-methoxy-N-methylacetamide

-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: iodine; magnesium / tetrahydrofuran / 25 - 50 °C 1.2: 40 - 50 °C 1.3: 0.25 h / 0 - 30 °C 2.1: toluene-4-sulfonic acid; 2,2'-azobis(isobutyronitrile); N-Bromosuccinimide / chloroform / 4 h / Reflux View Scheme | |
| Multi-step reaction with 2 steps 1.1: iodine; magnesium / tetrahydrofuran / 40 - 50 °C 1.2: 0.25 h / 0 - 30 °C 2.1: 2,2'-azobis(isobutyronitrile); N-Bromosuccinimide / toluene-4-sulfonic acid / dichloromethane / 4.75 h / 0 - 5 °C / Reflux View Scheme |
-
-
345-35-7
2-fluorobenzyl chloride

-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: tetrabutylammomium bromide / dichloromethane; water / 25 °C 2.1: sodium hydroxide; water / 5 - 95 °C 2.2: pH 2 - 3 3.1: magnesium; isopropyl bromide / toluene; tetrahydrofuran / 15 - 65 °C 3.2: 5 - 65 °C 4.1: dibenzoyl peroxide; N-Bromosuccinimide / ethyl acetate / 25 - 60 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: tetrabutylammomium bromide / dichloromethane; water / 25 °C 2.1: sodium hydroxide; water / 5 - 95 °C 2.2: pH 2 - 3 3.1: magnesium; isopropyl bromide / toluene; tetrahydrofuran / 15 - 65 °C 3.2: 5 - 65 °C 4.1: dibenzoyl peroxide; N-Bromosuccinimide / ethyl acetate / 25 - 60 °C View Scheme | |
| Multi-step reaction with 2 steps 1: magnesium; iodine / tetrahydrofuran; 2-methyltetrahydrofuran / 1.5 h / 40 - 45 °C / Inert atmosphere 2: N-Bromosuccinimide; toluene-4-sulfonic acid / acetonitrile / 24 h / 40 °C View Scheme | |
| Multi-step reaction with 2 steps 1: magnesium; iodine / tetrahydrofuran; toluene / 1.5 h / 40 - 45 °C / Inert atmosphere 2: N-Bromosuccinimide; toluene-4-sulfonic acid / acetonitrile / 24 h / 40 °C View Scheme |
-
-
326-62-5
2-fluorophenyl acetonitrile

-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: sodium hydroxide; water / 5 - 95 °C 1.2: pH 2 - 3 2.1: magnesium; isopropyl bromide / toluene; tetrahydrofuran / 15 - 65 °C 2.2: 5 - 65 °C 3.1: dibenzoyl peroxide; N-Bromosuccinimide / ethyl acetate / 25 - 60 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: sodium hydroxide; water / 5 - 95 °C 1.2: pH 2 - 3 2.1: magnesium; isopropyl bromide / toluene; tetrahydrofuran / 15 - 65 °C 2.2: 5 - 65 °C 3.1: dibenzoyl peroxide; N-Bromosuccinimide / ethyl acetate / 25 - 60 °C View Scheme |
-
-
4606-07-9
ethyl cyclopropylcarboxylate

-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone

| Conditions | Yield |
|---|---|
| Stage #1: With iodine; magnesium; isopropyl bromide In tetrahydrofuran at 65℃; for 3h; Stage #2: ethyl cyclopropylcarboxylate In tetrahydrofuran at 5 - 70℃; for 3.16667h; Stage #3: With N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile); toluene-4-sulfonic acid In chloroform at 28 - 65℃; for 4h; |

-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: triethylamine / dicyclohexyl-carbodiimide; benzotriazol-1-ol / dichloromethane / 6 h / 0 - 5 °C 2.1: iodine; magnesium / tetrahydrofuran / 40 - 50 °C 2.2: 0.25 h / 0 - 30 °C 3.1: 2,2'-azobis(isobutyronitrile); N-Bromosuccinimide / toluene-4-sulfonic acid / dichloromethane / 4.75 h / 0 - 5 °C / Reflux View Scheme |
-
-
462-06-6
fluorobenzene

-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: tin(IV) chloride / tetrahydrofuran / 4 h / 45 °C 2: dichloromethane / 4 h / 20 °C 3: bromine / tetrahydrofuran / 10 h / 20 °C View Scheme |
-
-
57486-67-6
methyl o-fluorophenylacetate

-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: pyridine / tetrahydrofuran / 6 h / 20 - 60 °C 2: 2,2'-azobis(isobutyronitrile); N-Bromosuccinimide / chloroform / 8 h / 20 °C View Scheme |
-
-
446-48-0
o-fluorobenzyl bromide

-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: magnesium / methyl iodide / diethyl ether / 20 °C 1.2: 20 °C 1.3: 0 °C 2.1: 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione; 2,2'-azobis(isobutyronitrile) / cyclohexane / 10 °C / Reflux View Scheme |
-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone

-
-
115473-15-9
5,6,7,7a-tetrahydro-thieno[3,2-c]pyridin-2(4H)-one hydrochloride

-
-
150322-38-6
5-(2-cyclopropyl-1-(2-fluorophenyl)-2-oxoethyl)-5,6,7,7a-tetrahydrothieno[3,2-c]pyridin-2(4H)-one

| Conditions | Yield |
|---|---|
| With ammonium bicarbonate In dichloromethane at 5 - 20℃; | 98% |
| With copper(l) iodide; sodium carbonate; XPhos In 1,4-dioxane at 60℃; for 3h; Reagent/catalyst; Temperature; Inert atmosphere; | 90.2% |
| With potassium phosphate; iodine In 1,4-dioxane at 80℃; for 4h; Temperature; Time; Reagent/catalyst; | 87.6% |
-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone

-
-
57-06-7
N-allyl isothiocyanate

-
-
589-16-2
4-aminoethylbenzene

| Conditions | Yield |
|---|---|
| With polyvinyl pyridine (PVP) In ethanol at 20℃; for 18h; regioselective reaction; | 96% |
| With 1-octyl-3-methylimidazole hexafluorophosphate at 20℃; for 1h; regioselective reaction; | 96% |

-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone

-
-
133477-39-1
2-[1-(2-naphthenyl)ethyledene]hydrazinecarbothioamide

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol for 0.333333h; Hantzsch Thiazole Synthesis; Reflux; | 96% |
-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone

-
-
106-49-0
p-toluidine

-
-
57-06-7
N-allyl isothiocyanate

| Conditions | Yield |
|---|---|
| With polyvinyl pyridine (PVP) In ethanol at 20℃; for 20h; Reagent/catalyst; Temperature; Time; regioselective reaction; | 95% |
| With 1-octyl-3-methylimidazole hexafluorophosphate at 20℃; for 1h; regioselective reaction; | 95% |

-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone

-
-
156-43-4
4-Ethoxyaniline

-
-
57-06-7
N-allyl isothiocyanate

| Conditions | Yield |
|---|---|
| With polyvinyl pyridine (PVP) In ethanol at 20℃; for 14h; regioselective reaction; | 95% |

-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone

-
-
304458-95-5
6-methoxy-tetral-4-one thiosemicarbazone

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol for 0.5h; Reagent/catalyst; Solvent; Temperature; Hantzsch Thiazole Synthesis; Reflux; | 95% |
-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone

-
-
156-43-4
4-Ethoxyaniline

-
-
57-06-7
N-allyl isothiocyanate

| Conditions | Yield |
|---|---|
| With 1-octyl-3-methylimidazole hexafluorophosphate at 20℃; for 0.75h; regioselective reaction; | 95% |

-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone

-
-
3689-17-6
2-(3,4-dihydronaphthalen-1(2H)-ylidene)hydrazinecarbothioamide

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol for 0.5h; Hantzsch Thiazole Synthesis; Reflux; | 94% |
-
-
5382-16-1
4-HYDROXYPIPERIDINE

-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone

| Conditions | Yield |
|---|---|
| With potassium carbonate In N-methyl-acetamide; water | 93% |
| With potassium carbonate In N-methyl-acetamide; water | 93% |
-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone

-
-
1263407-24-4
(5-(2-cyclopropyl-1-(2-fluorophenyl)-2-oxoethyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridin-2-yl)boronic acid

| Conditions | Yield |
|---|---|
| Stage #1: C7H10BNO2S*C7H8O3S With triethylamine In ethanol at 50℃; Inert atmosphere; Stage #2: 2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone In ethanol at 50℃; for 8h; | 93% |

-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone

-
-
1563931-94-1
3-(2-cyclopropyl-1-(2-fluorophenyl)-2-oxoethyl)thiazolidine-2,4-dione

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In N,N-dimethyl-formamide at 20℃; for 6h; | 93% |
-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone

-
-
104-94-9
4-methoxy-aniline

-
-
556-61-6
methyl thioisocyanate

| Conditions | Yield |
|---|---|
| With polyvinyl pyridine (PVP) In ethanol at 20℃; for 16h; regioselective reaction; | 93% |
| With 1-octyl-3-methylimidazole hexafluorophosphate at 20℃; for 1.5h; regioselective reaction; | 93% |

-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone

-
-
74227-66-0
1-indanone thiosemicarbazone

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol for 0.333333h; Hantzsch Thiazole Synthesis; Reflux; | 93% |
-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone

-
-
542-85-8
Ethyl isothiocyanate

-
-
589-16-2
4-aminoethylbenzene

| Conditions | Yield |
|---|---|
| With polyvinyl pyridine (PVP) In ethanol at 20℃; for 14h; regioselective reaction; | 92% |
| With 1-octyl-3-methylimidazole hexafluorophosphate at 20℃; for 1.5h; regioselective reaction; | 92% |

-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone

-
-
542-85-8
Ethyl isothiocyanate

-
-
156-43-4
4-Ethoxyaniline

| Conditions | Yield |
|---|---|
| With polyvinyl pyridine (PVP) In ethanol at 20℃; for 12h; regioselective reaction; | 91% |
| With 1-octyl-3-methylimidazole hexafluorophosphate at 20℃; for 2h; regioselective reaction; | 91% |

-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone

-
-
16546-08-0
1-(1-(4-methoxyphenyl)ethylidene)thiosemicarbazide

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol for 0.583333h; Hantzsch Thiazole Synthesis; Reflux; | 91% |
-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone

-
-
109904-37-2
5,6,7,7a-tetrahydrothieno[3,2-c]pyridine-2(4H)-one monohydrochloride

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
952340-38-4
2-(tert-butyldimethylsilyloxy)-5-(α-cyclopropylformyl-2-fluorobenzyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine

| Conditions | Yield |
|---|---|
| Stage #1: 5,6,7,7a-tetrahydrothieno[3,2-c]pyridine-2(4H)-one monohydrochloride With triethylamine In dichloromethane at 10℃; for 0.25h; Stage #2: tert-butyldimethylsilyl chloride In dichloromethane at 10℃; Stage #3: 2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone With triethylamine; sodium iodide In dichloromethane at 10 - 25℃; Temperature; | 90% |

-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone

-
-
71173-46-1
1-(1-(3,4-dimethoxyphenyl)ethylidene)thiosemicarbazide

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol for 0.666667h; Hantzsch Thiazole Synthesis; Reflux; | 90% |
-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone

-
-
104-94-9
4-methoxy-aniline

-
-
57-06-7
N-allyl isothiocyanate

| Conditions | Yield |
|---|---|
| With polyvinyl pyridine (PVP) In ethanol at 20℃; for 16h; regioselective reaction; | 89% |
| With 1-octyl-3-methylimidazole hexafluorophosphate at 20℃; for 0.75h; regioselective reaction; | 89% |

-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone

-
-
7410-54-0
2-(1-(4-tolyl)ethylidene)hydrazinecarbothioamide

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol for 0.583333h; Hantzsch Thiazole Synthesis; Reflux; | 89% |
-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone

-
-
106-40-1
4-bromo-aniline

-
-
57-06-7
N-allyl isothiocyanate

| Conditions | Yield |
|---|---|
| With polyvinyl pyridine (PVP) In ethanol at 20℃; for 20h; regioselective reaction; | 88% |
| With 1-octyl-3-methylimidazole hexafluorophosphate at 20℃; for 1.25h; regioselective reaction; | 88% |

-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone

-
-
5424-45-3
2-[1-(4-nitrophenyl)ethylidene]hydrazine-1-carbothioamide

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol for 0.5h; Hantzsch Thiazole Synthesis; Reflux; | 88% |

| Conditions | Yield |
|---|---|
| With potassium phosphate; iodine at 80℃; for 7h; Temperature; Reagent/catalyst; | 87.1% |
-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone

-
-
536-90-3
m-Anisidine

-
-
57-06-7
N-allyl isothiocyanate

| Conditions | Yield |
|---|---|
| With polyvinyl pyridine (PVP) In ethanol at 20℃; for 18h; regioselective reaction; | 87% |
| With 1-octyl-3-methylimidazole hexafluorophosphate at 20℃; for 1h; regioselective reaction; | 87% |

-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone

-
-
106-47-8
4-chloro-aniline

-
-
57-06-7
N-allyl isothiocyanate

| Conditions | Yield |
|---|---|
| With polyvinyl pyridine (PVP) In ethanol at 20℃; for 20h; regioselective reaction; | 87% |
| With 1-octyl-3-methylimidazole hexafluorophosphate at 20℃; for 1.25h; regioselective reaction; | 87% |

-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone

-
-
123-30-8
4-amino-phenol

-
-
57-06-7
N-allyl isothiocyanate

| Conditions | Yield |
|---|---|
| With polyvinyl pyridine (PVP) In ethanol at 20℃; for 24h; regioselective reaction; | 82% |
| With 1-octyl-3-methylimidazole hexafluorophosphate at 20℃; for 1h; regioselective reaction; | 82% |

-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone


| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 20℃; for 0.5h; | 81% |
-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone

-
-
853805-52-4
(E)-4-(acetylsulfanyl)-3-((1-[3-(ethoxycarbonyl)propyl]-1H-1,2,3-triazol-4-yl)methylidene)piperidine hydrogen trifluoroacetate

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 20℃; for 2.5h; | 77% |
-
-
204205-33-4
2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 0 - 20℃; for 1h; | 74% |
2-Bromo-2-(2-fluorophenyl)-1-cyclopropylethanone Chemical Properties
Molecule structure of 2-Bromo-2-(2-fluorophenyl)-1-cyclopropylethanone (CAS NO.204205-33-4):
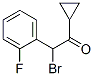
Molecular Formula: C11H10BrFO
Molecular Weight: 257.10 g/mol
Density: 1.574 g/cm3
Boiling Point: 293.012 °C at 760 mmHg
Flash Point: 131.009 °C
Index of Refraction: 1.592
Molar Refractivity: 55.256 cm3
Molar Volume: 163.331 cm3
Polarizability: 21.905×10-24 cm3
Surface Tension: 50.662 dyne/cm
Enthalpy of Vaporization: 53.255 kJ/mol
Vapour Pressure: 0.002 mmHg at 25 °C
InChI: InChI=1/C11H10BrFO/c12-10(11(14)7-5-6-7)8-3-1-2-4-9(8)13/h1-4,7,10H,5-6H2
InChIKey of 2-Bromo-2-(2-fluorophenyl)-1-cyclopropylethanone (CAS NO.204205-33-4): LMCZCCDXOZGIND-UHFFFAOYAC
2-Bromo-2-(2-fluorophenyl)-1-cyclopropylethanone Specification
2-Bromo-2-(2-fluorophenyl)-1-cyclopropylethanone (CAS NO.204205-33-4) is also named as 2-Bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone ; ethanone, 2-bromo-1-cyclopropyl-2-(2-fluorophenyl)- .
Related Products
- 2-Bromo-2-(2-fluorophenyl)-1-cyclopropylethanone
- 204206-08-6
- 2042-14-0
- 20422-68-8
- 2042-27-5
- 2042-37-7
- 20423-99-8
- 20424-00-4
- 204244-69-9
- 204244-79-1
- 204244-80-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View