-
Name
2-Bromovaleric acid
- EINECS 209-546-6
- CAS No. 584-93-0
- Article Data17
- CAS DataBase
- Density 1.518 g/cm3
- Solubility Insoluble in water, soluble in acetic acid, benzene, ether and other organic solvents
- Melting Point 252 °C (sublm)
- Formula C5H9BrO2
- Boiling Point 232.188 °C at 760 mmHg
- Molecular Weight 181.029
- Flash Point 94.225 °C
- Transport Information UN 3265 8/PG 2
- Appearance Clear pale yellow liquid
- Safety 26-27-28-36/37/39-45
- Risk Codes 23/24/25-34-22
-
Molecular Structure
-
Hazard Symbols
 T,
T,  C
C
- Synonyms 2-Bromopentanoic acid;Valeric acid, alpha-bromo-;alpha-Bromopentanoic acid;alpha-Bromovaleric acid;
- PSA 37.30000
- LogP 1.63460
Synthetic route

| Conditions | Yield |
|---|---|
| With bromine; phosphorus trichloride for 4.5h; Heating; | 91% |
| With N-Bromosuccinimide; sulfuric acid In trifluoroacetic acid at 85℃; for 16h; | 86% |
| With bromine; trichlorophosphate at 80 - 105℃; for 17h; | 72.6% |

| Conditions | Yield |
|---|---|
| With diethyl ether; bromine in direktem Sonnenlicht einwirken und erhitzt die hierbei erhaltene Brompropylmalonsaeure auf 145; |

| Conditions | Yield |
|---|---|
| With hydrogen bromide; iron(II) sulfate |

| Conditions | Yield |
|---|---|
| With bromine at 90℃; for 16h; 2) 100 deg C, 0.5 h; Yield given; |

| Conditions | Yield |
|---|---|
| With hydrogen bromide; sodium nitrite In water |

| Conditions | Yield |
|---|---|
| With 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide; triethylamine In dichloromethane at 15℃; for 2h; | 90% |
-
-
584-93-0
2-Bromovaleric acid


| Conditions | Yield |
|---|---|
| With thiourea | A n/a B 87% |

| Conditions | Yield |
|---|---|
| With sulfuric acid Reflux; | 87% |
-
-
584-93-0
2-Bromovaleric acid

-
-
23336-68-7
triethylammonium-2-N-morpholino-dithiocarbamate

-
-
181036-27-1
2-N-morpholino-dithiocarbamoyl-pentanoic acid

| Conditions | Yield |
|---|---|
| In benzene at 20℃; for 16h; | 85% |
| In Petroleum ether for 6h; Ambient temperature; Yield given; |
-
-
584-93-0
2-Bromovaleric acid

-
-
99-52-5
2-methyl-4-nitro-benzenamine

-
-
83473-12-5
2-Bromo-pentanoic acid (2-methyl-4-nitro-phenyl)-amide

| Conditions | Yield |
|---|---|
| With phosphorus trichloride In benzene for 3h; Heating; | 84% |
-
-
584-93-0
2-Bromovaleric acid

-
-
188717-79-5
2-(4-chloro-5-methyl-2-mercaptobenzenesulfonylimino)hexahydropyrimidine

-
-
188718-92-5
2-[5-Chloro-4-methyl-2-(tetrahydro-pyrimidin-2-ylidenesulfamoyl)-phenylsulfanyl]-pentanoic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide; sodium carbonate In water at 80 - 85℃; for 2h; | 78% |
-
-
584-93-0
2-Bromovaleric acid

-
-
160985-01-3
1-(4-chloro-5-cyano-2-mercaptobenzenesulphonyl)-2-imidazolidinone

| Conditions | Yield |
|---|---|
| With sodium hydroxide; sodium carbonate In water at 40 - 80℃; for 6.5h; | 76% |
-
-
584-93-0
2-Bromovaleric acid

-
-
16947-63-0
2,6-dimethyl-4-nitroaniline

| Conditions | Yield |
|---|---|
| With phosphorus trichloride In benzene for 3h; Heating; | 73% |
-
-
584-93-0
2-Bromovaleric acid

-
-
160985-00-2
1-(5-cyano-2-mercaptobenzenesulphonyl)-2-imidazolidinone

| Conditions | Yield |
|---|---|
| With sodium hydroxide; sodium carbonate In water at 40 - 80℃; for 6.5h; | 73% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-Bromovaleric acid With pivaloyl chloride; triethylamine In tetrahydrofuran at 0℃; for 4h; Inert atmosphere; Stage #2: dibenzylamine With lithium chloride In tetrahydrofuran at 20℃; for 16h; Inert atmosphere; | 71% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-Bromovaleric acid With pivaloyl chloride; triethylamine In tetrahydrofuran at 0℃; for 4h; Inert atmosphere; Stage #2: piperidine With lithium chloride In tetrahydrofuran at 20℃; for 16h; Inert atmosphere; | 69% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-Bromovaleric acid With oxalyl dichloride; N,N-dimethyl-formamide In 1,2-dichloro-ethane at 0 - 20℃; for 5h; Stage #2: 5-bromo-2-pyridylamine With N-ethyl-N,N-diisopropylamine In 1,2-dichloro-ethane at 20℃; for 1.16667h; | 65% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-Bromovaleric acid With pivaloyl chloride; triethylamine In tetrahydrofuran at 0℃; for 4h; Inert atmosphere; Stage #2: diethylamine With lithium chloride In tetrahydrofuran at 20℃; for 16h; Inert atmosphere; | 64% |
-
-
584-93-0
2-Bromovaleric acid

-
-
188717-81-9
2-(4-chloro-5-methyl-2-mercaptobenzenesulfonylimino)-5,5-dimethylhexahydropyrimidine

-
-
188718-95-8
2-[5-Chloro-2-(5,5-dimethyl-tetrahydro-pyrimidin-2-ylidenesulfamoyl)-4-methyl-phenylsulfanyl]-pentanoic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide; sodium carbonate In water at 80 - 85℃; for 2h; | 60% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 140℃; for 0.333333h; Microwave irradiation; | 58% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-Bromovaleric acid With oxalyl dichloride In dichloromethane at 40℃; for 2h; Inert atmosphere; Stage #2: diisopropylamine With lithium chloride In tetrahydrofuran at 20℃; for 1.5h; Inert atmosphere; | 57% |
-
-
584-93-0
2-Bromovaleric acid

-
-
168698-63-3
2-[5-Chloro-4-methyl-2-(2-oxo-imidazolidine-1-sulfonyl)-phenylsulfanyl]-pentanoic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide; sodium carbonate In water at 40 - 80℃; for 6.5h; | 55% |

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 20℃; for 10h; | 54% |
-
-
584-93-0
2-Bromovaleric acid

-
-
188717-83-1
4-Chloro-N-[4-ethyl-tetrahydro-pyrimidin-(2E)-ylidene]-2-mercapto-5-methyl-benzenesulfonamide

-
-
188718-97-0
2-{5-Chloro-2-[4-ethyl-tetrahydro-pyrimidin-(2E)-ylidenesulfamoyl]-4-methyl-phenylsulfanyl}-pentanoic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide; sodium carbonate In water at 80 - 85℃; for 2h; | 53% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-Bromovaleric acid With oxalyl dichloride In dichloromethane at 40℃; for 2h; Inert atmosphere; Stage #2: N-Benzylaniline With lithium chloride In tetrahydrofuran at 20℃; for 1.5h; Inert atmosphere; | 53% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 0 - 100℃; for 20h; | 51.9% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-Bromovaleric acid With pivaloyl chloride; triethylamine In tetrahydrofuran at 0℃; for 4h; Inert atmosphere; Stage #2: morpholine With lithium chloride In tetrahydrofuran at 20℃; for 16h; Inert atmosphere; | 46% |

| Conditions | Yield |
|---|---|
| With phosphate buffer; tris[3-(2-methoxyethoxy)propyl]stannane In water Irradiation; or with ACVA initiation; | 37% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 140℃; for 0.333333h; Microwave irradiation; | 37% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 140℃; for 0.333333h; Microwave irradiation; | 36% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-Bromovaleric acid With pivaloyl chloride; triethylamine In tetrahydrofuran at 0℃; for 4h; Inert atmosphere; Stage #2: 2.6-dimethylpiperidine With lithium chloride In tetrahydrofuran at 20℃; for 16h; Inert atmosphere; | 32% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-Bromovaleric acid With pivaloyl chloride; triethylamine In tetrahydrofuran at 0℃; for 4h; Inert atmosphere; Stage #2: N-cyclohexyl-cyclohexanamine With lithium chloride In tetrahydrofuran at 20℃; for 16h; Inert atmosphere; | 28% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-Bromovaleric acid With pivaloyl chloride; triethylamine In tetrahydrofuran at 0℃; for 4h; Inert atmosphere; Stage #2: benzylamine With lithium chloride In tetrahydrofuran at 20℃; for 16h; Inert atmosphere; | 19% |
2-Bromovaleric acid Uses
&alpha-Bromovaleric acid (584-93-0)
2-Bromovaleric acid Consensus Reports
Reported in EPA TSCA Inventory.
2-Bromovaleric acid Specification
The 2-Bromovaleric acid, with the CAS registry number 584-93-0, is also known as alpha-Bromopentanoic acid. It belongs to the product categories of Organic acids; C1 to C5; Carbonyl Compounds; Carboxylic Acids. Its EINECS number is 209-546-6. This chemical's molecular formula is C5H9BrO2 and molecular weight is 181.03. What's more, its systematic name is 2-Bromopentanoic acid. It can be used as pharmaceutical intermediates.
Physical properties of 2-Bromovaleric acid are: (1)ACD/LogP: 1.884; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -0.62; (4)ACD/LogD (pH 7.4): -1.78; (5)ACD/BCF (pH 5.5): 1.00; (6)ACD/BCF (pH 7.4): 1.00; (7)ACD/KOC (pH 5.5): 1.00; (8)ACD/KOC (pH 7.4): 1.00; (9)#H bond acceptors: 2; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 3; (12)Polar Surface Area: 37.3 Å2; (13)Index of Refraction: 1.49; (14)Molar Refractivity: 34.468 cm3; (15)Molar Volume: 119.253 cm3; (16)Polarizability: 13.664×10-24cm3; (17)Surface Tension: 41.34 dyne/cm; (18)Density: 1.518 g/cm3; (19)Flash Point: 94.225 °C; (20)Enthalpy of Vaporization: 51.647 kJ/mol; (21)Boiling Point: 232.188 °C at 760 mmHg; (22)Vapour Pressure: 0.02 mmHg at 25°C.
Preparation: this chemical can be prepared by pentanoic acid at the temperature of 85 °C. This reaction will need reagents NBS, conc. H2SO4 and solvent trifluoroacetic acid with the reaction time of 16 hours. The yield is about 86%.

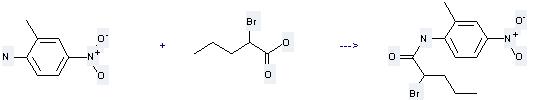
Uses of 2-Bromovaleric acid: it can be used to produce 2-bromo-pentanoic acid (2-methyl-4-nitro-phenyl)-amide by heating. It will need reagent PCl3 and solvent benzene with the reaction time of 3 hours. The yield is about 84%.
When you are using this chemical, please be cautious about it as the following:
This chemical is toxic by inhalation, in contact with skin and if swallowed. It is harmful if swallowed and can cause burns. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. You must take off immediately all contaminated clothing. After contact with skin, you should wash immediately with plenty of ... (to be specified by the manufacturer). When using it, you need to wear suitable protective clothing, gloves and eye/face protection. In case of accident or if you feel unwell, you should seek medical advice immediately (show the label where possible).
You can still convert the following datas into molecular structure:
(1)SMILES: BrC(C(=O)O)CCC
(2)Std. InChI: InChI=1S/C5H9BrO2/c1-2-3-4(6)5(7)8/h4H,2-3H2,1H3,(H,7,8)
(3)Std. InChIKey: WMFATTFQNRPXBQ-UHFFFAOYSA-N
Related Products
- 2-Bromovaleric acid
- 58493-21-3
- 58493-71-3
- 58498-61-6
- 58501-21-6
- 5850-13-5
- 5850-15-7
- 5850-16-8
- 58502-11-7
- 5850-35-1
- 5850-39-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View