-
Name
2-CHLORO-4'-HYDROXYACETOPHENONE
- EINECS 228-613-0
- CAS No. 6305-04-0
- Article Data24
- CAS DataBase
- Density 1.304 g/cm3
- Solubility
- Melting Point 145-146 °C
- Formula C8H7 Cl O2
- Boiling Point 337.1 ºC at 760 mmHg
- Molecular Weight 170.595
- Flash Point 157.7 ºC
- Transport Information
- Appearance
- Safety A poison by ingestion. A severe skin and eye irritant. When heated to decomposition it emits toxic vapors of Cl−.
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Acetophenone,2-chloro-4'-hydroxy- (6CI,7CI); 2-Chloro-1-(4-hydroxyphenyl)ethanone;2-Chloro-4'-hydroxyacetophenone; 4-Hydroxy-a-chloroacetophenone; 4-Hydroxyphenacyl chloride;4'-Hydroxy-2-chloroacetophenone; Chlorophenacyle; NSC 41671;p-(Chloroacetyl)phenol; p-Hydroxyphenacyl chloride; w-Chloro-4-hydroxyacetophenone
- PSA 37.30000
- LogP 1.81370
Synthetic route

| Conditions | Yield |
|---|---|
| With N,N,N-trimethylbenzenemethanaminium dichloroiodate In methanol; 1,2-dichloro-ethane for 10h; Heating; | 95% |
| With 2,2,3,4,5,6-hexachloro-cyclohexa-2,4-dien-1-one In ethanol for 5h; Heating; | 77% |
| With hydrogenchloride; ammonium nitrate; iodine; oxygen In water; acetonitrile at 60℃; for 22h; Green chemistry; chemoselective reaction; | 64% |
| With N-chloro-succinimide; toluene-4-sulfonic acid In acetonitrile at 40℃; | 45% |

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride In dichloromethane at 0 - 30℃; for 7h; Solvent; | 94% |
| With aluminium trichloride In various solvent(s) at 70℃; for 5h; | 50% |
| Stage #1: chloroacetyl chloride; phenol; aluminum (III) chloride In 1,2-dichloro-ethane at 0 - 65℃; for 13h; Stage #2: With hydrogenchloride In dichloromethane; water | 15% |
-
-
868746-76-3
2-chloro-1-(4-chloroacetoxy-phenyl)-ethanone

-
-
6305-04-0
p-hydroxyphenacyl chloride

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol; water at 0 - 20℃; for 0.5h; | 85% |
-
-
620-73-5
phenyl chloroacetate

-
A
-
53074-73-0
2-(2-chloroacetyl)phenol

-
B
-
6305-04-0
p-hydroxyphenacyl chloride

| Conditions | Yield |
|---|---|
| With beryllium(II) chloride at 130 - 140℃; | |
| With aluminium trichloride at 120℃; |

| Conditions | Yield |
|---|---|
| With aluminium trichloride |
-
-
100-66-3
methoxybenzene

-
-
79-04-9
chloroacetyl chloride

-
A
-
53074-73-0
2-(2-chloroacetyl)phenol

-
B
-
6305-04-0
p-hydroxyphenacyl chloride

| Conditions | Yield |
|---|---|
| With aluminium trichloride; 1,1,2,2-tetrachloroethane | |
| With aluminium trichloride; 1,1,2,2-tetrachloroethane |


| Conditions | Yield |
|---|---|
| With aluminium trichloride; Petroleum ether | |
| With aluminium trichloride | |
| With carbon disulfide; aluminium trichloride | |
| With aluminium trichloride |

-
-
6305-04-0
p-hydroxyphenacyl chloride

| Conditions | Yield |
|---|---|
| at 110℃; |
-
-
620-73-5
phenyl chloroacetate

-
-
7446-70-0
aluminium trichloride

-
A
-
6305-04-0
p-hydroxyphenacyl chloride

| Conditions | Yield |
|---|---|
| at 120℃; |


| Conditions | Yield |
|---|---|
| With aluminium trichloride at 120℃; |

| Conditions | Yield |
|---|---|
| With beryllium(II) chloride at 130 - 140℃; |

| Conditions | Yield |
|---|---|
| With indium(I) bromide In tetrahydrofuran at 20℃; for 24h; |

| Conditions | Yield |
|---|---|
| With aluminium trichloride In n-heptane for 2.5h; Heating; | 9.5 g |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: AlCl3 / heptane / 1.5 h / 35 °C 2: 9.5 g / AlCl3 / heptane / 2.5 h / Heating View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: HCl / methanol / 10 °C 2: 110 °C View Scheme |
-
-
6305-04-0
p-hydroxyphenacyl chloride

-
-
100-39-0
benzyl bromide

-
-
63365-56-0
1-(4-(benzyloxy)phenyl)-2-chloroethane-1-one

| Conditions | Yield |
|---|---|
| Stage #1: p-hydroxyphenacyl chloride With potassium carbonate In N,N-dimethyl-formamide at 20 - 30℃; for 0.5h; Stage #2: benzyl bromide In N,N-dimethyl-formamide at 30 - 40℃; for 4h; Solvent; Reagent/catalyst; | 97.5% |
-
-
6305-04-0
p-hydroxyphenacyl chloride

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
130798-41-3
1-(4-(tert-butyldimethylsilyloxy)phenyl)-2-chloroethanone

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide at 23℃; for 0.05h; | 97% |

| Conditions | Yield |
|---|---|
| With ammonium propionate; propionic acid at 190℃; for 30h; | 92% |
-
-
1450-76-6
1-(2-hydroxy-5-nitrophenyl)ethanone

-
-
6305-04-0
p-hydroxyphenacyl chloride

-
-
141645-21-8
2-(4-hydroxy benzoyl) 3-methyl 5-nitro benzofuran

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In dichloromethane at 20℃; for 4h; Solvent; Molecular sieve; Inert atmosphere; | 91.1% |
-
-
917-64-6
methyl magnesium iodide

-
-
6305-04-0
p-hydroxyphenacyl chloride

-
A
-
770-39-8
4-methoxyphenylacetone

-
B
-
98815-43-1
1-[(4-hydroxy)phenyl]-2-methyl-2-propanol

-
C
-
98815-45-3
1-chloro-2-(4-hydroxyphenyl)propan-2-ol

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 2h; Title compound not separated from byproducts; | A 5% B 90% C n/a |

-
-
917-64-6
methyl magnesium iodide

-
-
6305-04-0
p-hydroxyphenacyl chloride

-
A
-
770-39-8
4-methoxyphenylacetone

-
B
-
98815-45-3
1-chloro-2-(4-hydroxyphenyl)propan-2-ol

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 2h; Title compound not separated from byproducts; | A 90% B 90% |

-
-
6305-04-0
p-hydroxyphenacyl chloride

-
-
124-41-4
sodium methylate

-
-
32136-81-5
alpha-methoxy-4-hydroxyacetophenone

| Conditions | Yield |
|---|---|
| In methanol for 24h; Ambient temperature; | 90% |
| In methanol pH=8; pH-value; | 18.3 g |

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In dichloromethane at 20℃; for 3h; Solvent; Molecular sieve; Inert atmosphere; | 88.54% |
-
-
7338-80-9
6-(phenylmethyl)-3,4-dihydro-3-thioxo-1,2,4-triazin-5(2H)-one

-
-
6305-04-0
p-hydroxyphenacyl chloride

| Conditions | Yield |
|---|---|
| With sodium acetate; acetic acid Reflux; | 85% |
| With sodium acetate In acetic acid Reflux; | 79.2% |
| With sodium acetate; acetic acid |

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In dichloromethane at 20℃; for 3h; Solvent; Molecular sieve; Inert atmosphere; | 81% |
-
-
6305-04-0
p-hydroxyphenacyl chloride

-
-
112517-73-4
6-[(4-methoxyphenyl)methyl]-3,4-dihydro-3-thioxo-1,2,4-triazin-5(2H)-one

| Conditions | Yield |
|---|---|
| With sodium acetate In acetic acid Reflux; | 80.1% |
| With sodium acetate; acetic acid Reflux; | 64% |
| With sodium acetate In acetic acid Reflux; | 62.7% |
| With sodium acetate; acetic acid | |
| With sodium acetate In acetic acid Reflux; |
-
-
6305-04-0
p-hydroxyphenacyl chloride

-
-
143540-96-9
5-p-Tolyloxymethyl-2,4-dihydro-[1,2,4]triazole-3-thione

| Conditions | Yield |
|---|---|
| In propan-1-ol for 20h; Heating; | 75% |
-
-
6305-04-0
p-hydroxyphenacyl chloride

-
A
-
156-38-7
4-hydroxyphenylacetate

-
B
-
108791-64-6
1,4-bis(4-hydroxyphenyl)butan-1,4-dione

-
C
-
99-93-4
4-Hydroxyacetophenone

| Conditions | Yield |
|---|---|
| With methyloxirane In water; acetonitrile for 4h; Irradiation; | A 75% B 10% C 5% |

-
-
6305-04-0
p-hydroxyphenacyl chloride

-
-
75-31-0
isopropylamine

-
-
69716-74-1
1-(4-hydroxy-phenyl)-2-isopropylamino-ethanone; hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: p-hydroxyphenacyl chloride; isopropylamine In methanol at 20℃; for 3h; Stage #2: With hydrogenchloride In methanol; water | 75% |
-
-
6305-04-0
p-hydroxyphenacyl chloride

-
-
93703-24-3
3-methyl-8-bromoxanthine

-
-
1192215-81-8
8-bromo-3,7-dihydro-7-[2-(4-hydroxyphenyl)-2-oxoethyl]-3-methyl-1H-purine-2,6-dione

| Conditions | Yield |
|---|---|
| Stage #1: 3-methyl-8-bromoxanthine With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 25℃; for 0.0833333h; Inert atmosphere; Stage #2: p-hydroxyphenacyl chloride In N,N-dimethyl-formamide at 25℃; Inert atmosphere; | 74% |
-
-
6305-04-0
p-hydroxyphenacyl chloride

-
-
30186-18-6
4'-bromo-2'-hydroxyacetophenone

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In dichloromethane at 20℃; for 3h; Solvent; Molecular sieve; Inert atmosphere; | 73.2% |
-
-
6305-04-0
p-hydroxyphenacyl chloride

-
-
202475-60-3
WHI-P131

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 18h; | 73% |
-
-
6305-04-0
p-hydroxyphenacyl chloride

-
-
143540-95-8
5-o-Tolyloxymethyl-2,4-dihydro-[1,2,4]triazole-3-thione

| Conditions | Yield |
|---|---|
| In propan-1-ol for 20h; Heating; | 72% |
-
-
6305-04-0
p-hydroxyphenacyl chloride

-
-
118-93-4
o-hydroxyacetophenone

-
-
32730-17-9
(4-hydroxy-phenyl)-(3-methyl-benzofuran-2-yl)-methanone

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In dichloromethane at 20℃; for 4h; Solvent; Molecular sieve; Inert atmosphere; | 72% |
-
-
6305-04-0
p-hydroxyphenacyl chloride

-
-
78171-88-7
5-Phenoxymethyl-2,4-dihydro-[1,2,4]triazole-3-thione

| Conditions | Yield |
|---|---|
| In propan-1-ol for 20h; Heating; | 70% |
-
-
6305-04-0
p-hydroxyphenacyl chloride

-
-
372164-14-2
5-m-Tolyloxymethyl-2,4-dihydro-[1,2,4]triazole-3-thione

| Conditions | Yield |
|---|---|
| In propan-1-ol for 20h; Heating; | 70% |
-
-
6305-04-0
p-hydroxyphenacyl chloride

-
-
33401-57-9
6-(4-chlorobenzyl)-3-thioxo-3,4-dihydro-[1,2,4]-triazin-5(2H)-one

| Conditions | Yield |
|---|---|
| With sodium acetate; acetic acid Reflux; | 65% |
| With sodium acetate In acetic acid Reflux; | 65.4% |
| With sodium acetate; acetic acid |
-
-
447-00-7
6-phenyl-1,2,4-triazine-3(2H)-thione-5(4H)-one

-
-
6305-04-0
p-hydroxyphenacyl chloride

-
-
1186078-85-2
3-(4-hydroxyphenyl)-6-phenyl-7H-thiazolo[3,2-b][1,2,4]triazin-7-one

| Conditions | Yield |
|---|---|
| With acetic acid for 12h; Reflux; | 60% |
-
-
6305-04-0
p-hydroxyphenacyl chloride

-
-
64-19-7
acetic acid

-
-
20816-46-0
α,α-dimethyl-p-hydroxyphenacyl acetate

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile for 3h; Heating; | 50% |
| With triethylamine In acetonitrile for 3h; Heating; | 40% |
-
-
6305-04-0
p-hydroxyphenacyl chloride

| Conditions | Yield |
|---|---|
| With sodium acetate In acetic acid Reflux; | 42.8% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; sodium iodide for 3.5h; Heating; | 38% |
-
-
6305-04-0
p-hydroxyphenacyl chloride

-
-
79-19-6
thiosemicarbazide

-
-
74495-46-8
2-amino-5-(p-hydroxyphenyl)-1,3,4-thiadiazine hydrochloride

| Conditions | Yield |
|---|---|
| In ethanol Heating; | 27% |
2-Chloro-4'-hydroxyacetophenone Chemical Properties
IUPAC Name: 2-Chlor-1-(4-hydroxyphenyl)ethanon
The MF of 2-Chlor-1-(4-hydroxyphenyl)ethanon (6305-04-0) is C8H7ClO2.
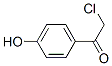
The MW of 2-Chlor-1-(4-hydroxyphenyl)ethanon (6305-04-0) is 170.59298.
Synonyms of 2-Chlor-1-(4-hydroxyphenyl)ethanon (6305-04-0): 2-Chloro-1-(4-hydroxyphenyl)ethanone ; Ethanone, 2-chloro-1- (4-hydroxyphenyl)- ; 2-Chloro-1-(4-hydroxyphenyl)ethan-1-one ; 2-Chloro-4'-hydroxyacetophenone
Index of Refraction: 1.571
EINECS: 228-613-0
Density: 1.304 g/ml
Flash Point: 157.7 °C
Boiling Point: 337.1 °C
2-Chloro-4'-hydroxyacetophenone Toxicity Data With Reference
| 1. | skn-rbt 500 mg SEV | JACTDZ Journal of the American College of Toxicology. 12 (1993),581. | ||
| 2. | eye-rbt 10 mg SEV | JACTDZ Journal of the American College of Toxicology. 12 (1993),581. | ||
| 3. | orl-rat LD50:230 mg/kg | JACTDZ Journal of the American College of Toxicology. 12 (1993),581. | ||
| 4. | skn-rbt LD50:>8 g/kg | JACTDZ Journal of the American College of Toxicology. 12 (1993),581. |
2-Chloro-4'-hydroxyacetophenone Safety Profile
A poison by ingestion. A severe skin and eye irritant. When heated to decomposition it emits toxic vapors of Cl−.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View