-
Name
2-Ethyl-4-methylimidazole
- EINECS 213-234-5
- CAS No. 931-36-2
- Article Data12
- CAS DataBase
- Density 1 g/cm3
- Solubility 210 g/L (20 ºC)
- Melting Point 47-54 °C(lit.)
- Formula C6H10N2
- Boiling Point 276 °C at 760 mmHg
- Molecular Weight 110.159
- Flash Point 137.8 °C
- Transport Information
- Appearance clear yellow viscous liquid after melting
- Safety 26-39
- Risk Codes 22-41
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, Xi
Xi
- Synonyms Imicure EMI 24;Epicure EMI 24;1H-Imidazole,2-ethyl-4-methyl-;2-ethyl-4-methyl-3H-imidazole;Imidazole, 2-ethyl-4-methyl-;4-Methyl-2-ethylimidazole;Imidazole, 2-ethyl-4-methyl- (8CI);2-Ethyl-4-methyl imidazole;2-Ethyl-4-methyl-1H-imidazole;EMI 70;Curezol 2E4MZ;1H-Imidazole, 2-ethyl-4-methyl-;
- PSA 28.68000
- LogP 1.28050
Synthetic route

| Conditions | Yield |
|---|---|
| With potassium hydroxide In water at 190℃; for 0.25h; | 90.4% |
| With 5%-palladium/activated carbon at 140℃; for 10h; Reagent/catalyst; Temperature; | 86.8% |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; copper diacetate |
-
-
66675-19-2
N,N'-(methyl-ethenediyl)-bis-benzamide

-
-
123-62-6
propionic acid anhydride

-
-
931-36-2
2-ethyl-4-methyl-1H-imidazole

| Conditions | Yield |
|---|---|
| at 180℃; im Rohr; |
-
-
802294-64-0
propionic acid

-
-
78-90-0, 10424-38-1
1,2-diaminopropan

-
-
931-36-2
2-ethyl-4-methyl-1H-imidazole

| Conditions | Yield |
|---|---|
| With hydrogen; Pt/Al2O3; AP-64K platinum-alumina catalyst at 400℃; |
-
-
931-36-2
2-ethyl-4-methyl-1H-imidazole

-
-
403-43-0
4-fluorobenzoyl chloride

-
-
90172-65-9
2-ethyl-1-(p-fluorophenylcarbonyl)-4-methylimidazole

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane In chloroform-d1 | 100% |
-
-
50-00-0
formaldehyd

-
-
931-36-2
2-ethyl-4-methyl-1H-imidazole

-
-
94887-76-0
2-ethyl-4-methyl-5-hydroxymethyl-imidazole

| Conditions | Yield |
|---|---|
| With potassium hydroxide In water at 15 - 25℃; for 28h; pH=12.5; Reagent/catalyst; Time; Mannich Aminomethylation; | 98% |

| Conditions | Yield |
|---|---|
| With potassium carbonate sesquihydrate In chloroform at 20℃; for 144h; | 98% |

| Conditions | Yield |
|---|---|
| In ethyl acetate Reflux; | 93% |
-
-
931-36-2
2-ethyl-4-methyl-1H-imidazole

| Conditions | Yield |
|---|---|
| With boric acid In methanol; water for 1h; | 92% |

| Conditions | Yield |
|---|---|
| With C22H48N4(4+)*4HO(1-) In neat (no solvent) at 20℃; for 0.75h; Michael Addition; | 92% |

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In 1,4-dioxane at 60℃; for 2h; | 91% |
| With potassium carbonate In acetonitrile at 20℃; for 5h; |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In dimethyl sulfoxide at 120℃; Inert atmosphere; diastereoselective reaction; | 89% |
-
-
931-36-2
2-ethyl-4-methyl-1H-imidazole

-
-
107-13-1
acrylonitrile

-
-
23996-25-0
3-(2-ethyl-4-methyl-1H-imidazol-1-yl)propanenitrile

| Conditions | Yield |
|---|---|
| With C22H48N4(4+)*4HO(1-) In neat (no solvent) at 20℃; for 1.16667h; Michael Addition; | 87% |
-
-
931-36-2
2-ethyl-4-methyl-1H-imidazole

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
1299491-23-8
N-(tert-butoxycarbonyl)-2-ethyl-4-methylimidazole

| Conditions | Yield |
|---|---|
| With dmap In N,N-dimethyl-formamide for 1h; | 86% |
-
-
50-00-0
formaldehyd

-
-
931-36-2
2-ethyl-4-methyl-1H-imidazole

-
-
95684-24-5
bis(2-ethyl-4-methyl-imidazol-5-yl)methane

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol at 20℃; for 0.0833333h; pH=14; | 85% |
| In methanol | 85% |
-
-
50-00-0
formaldehyd

-
-
931-36-2
2-ethyl-4-methyl-1H-imidazole

-
-
95684-24-5
4,4′-methanediylbis(2-ethyl-5-methyl-1H-imidazole)

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol; water at 20℃; for 24h; | 85% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-ethyl-4-methyl-1H-imidazole; glycine With 1H-imidazole In water for 0.166667h; Stage #2: formaldehyd In water for 0.25h; Stage #3: With triethylamine In water at 20℃; for 168h; pH=11 - 12; | 85% |


| Conditions | Yield |
|---|---|
| Stage #1: 2-ethyl-4-methyl-1H-imidazole With sodium hydride In N,N-dimethyl-formamide; mineral oil at 0℃; for 0.5h; Stage #2: 2-chloropyrimidine In N,N-dimethyl-formamide; mineral oil at 130℃; | 85% |
-
-
931-36-2
2-ethyl-4-methyl-1H-imidazole

-
-
1096706-56-7
2-bromo-N-(2-bromoethyl)-N-(2,4-dichlorobenzyl)ethanamine

-
-
1096706-50-1
N-(2,4-dichlorobenzyl)-2-(2-ethyl-4-methyl-1H-imidazol-1-yl)-N-(2-(2-ethyl-4-methyl-1H-imidazol-1-yl)ethyl)ethanamine

| Conditions | Yield |
|---|---|
| Stage #1: 2-ethyl-4-methyl-1H-imidazole With sodium hydride In tetrahydrofuran at 60℃; for 1h; Stage #2: 2-bromo-N-(2-bromoethyl)-N-(2,4-dichlorobenzyl)ethanamine In tetrahydrofuran at 20 - 60℃; | 84.8% |
-
-
931-36-2
2-ethyl-4-methyl-1H-imidazole

-
-
84255-42-5
2-ethyl-4-methylimidazole-5-dithiocarboxylic acid

| Conditions | Yield |
|---|---|
| With carbon disulfide; sodium hydroxide In dimethyl sulfoxide | 83% |
-
-
931-36-2
2-ethyl-4-methyl-1H-imidazole

-
-
768-60-5
4-methoxyphenylacetylen

-
-
1383663-42-0
(Z)-2-ethyl-1-(4-methoxystyryl)-4-methyl-1H-imidazole

| Conditions | Yield |
|---|---|
| With potassium hydroxide In dimethyl sulfoxide at 120℃; for 2h; Inert atmosphere; stereoselective reaction; | 82% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; 1-Hydroxymethyl-1H-benzotriazole; potassium tert-butylate In dimethyl sulfoxide at 120℃; for 18h; Inert atmosphere; | 82% |
-
-
931-36-2
2-ethyl-4-methyl-1H-imidazole

-
-
137768-73-1
1-isothiocyanatopropa-1,2-diene

-
A
-
1037456-35-1
2-(2-ethyl-4-methylimidazol-1-yl)-5-methylthiazole

-
B
-
1037456-79-3
2-(2-ethyl-4-methylimidazol-1-yl)-5-methylene-4,5-dihydrothiazole

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 1h; | A 80% B 10% |


| Conditions | Yield |
|---|---|
| With sodium hydroxide In dimethyl sulfoxide at 20℃; | 80% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In dimethyl sulfoxide at 120℃; for 1.5h; Schlenk technique; Inert atmosphere; | 80% |
-
-
1037456-15-7
3-isothiocyanato-4-methoxybuta-1,2-diene

-
-
931-36-2
2-ethyl-4-methyl-1H-imidazole

-
A
-
1037456-37-3
2-(2-ethyl-4-methylimidazol-1-yl)-4-methoxymethyl-5-methylthiazole

-
B
-
1037456-81-7
2-(2-ethyl-4-methylimidazol-1-yl)-4-methoxymethyl-5-methylene-4,5-dihydrothiazole

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 1h; | A 79% B 3% |

-
-
931-36-2
2-ethyl-4-methyl-1H-imidazole

-
-
1073-06-9
3-fluorobromobenzene

-
-
109-63-7
boron trifluoride diethyl etherate

| Conditions | Yield |
|---|---|
| Stage #1: 3-fluorobromobenzene; boron trifluoride diethyl etherate With magnesium In tetrahydrofuran for 1h; Reflux; Stage #2: 2-ethyl-4-methyl-1H-imidazole With hydrogenchloride In tetrahydrofuran; water at 20℃; for 0.5h; | 79% |


| Conditions | Yield |
|---|---|
| With methanol at 20℃; for 15h; Michael Addition; diastereoselective reaction; | 78% |
-
-
931-36-2
2-ethyl-4-methyl-1H-imidazole

-
-
105-58-8
Diethyl carbonate

-
-
56468-51-0
1-ethyl-2-ethyl-4-methylimidazole

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In dimethyl sulfoxide at 90℃; for 10h; | 78% |

| Conditions | Yield |
|---|---|
| for 2h; Heating; | 76% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In chloroform for 8h; Reflux; | 75.35% |
-
-
931-36-2
2-ethyl-4-methyl-1H-imidazole

-
-
197958-29-5
pyridin-2-ylboronic acid

-
-
1167443-57-3
2-(2-ethyl-4-methyl-1H-imidazol-1-yl)pyridine

| Conditions | Yield |
|---|---|
| With 2Na(1+)*CuC6H4(NCHC6H3OO3S)2(2-)=CuC6H4(NCHC6H3ONaO3S)2 In water at 100℃; for 6h; | 75% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In water at 80℃; for 72h; | 75% |
2-Ethyl-4-methylimidazole Specification
The 2-Ethyl-4-methylimidazole with CAS registry number of 931-36-2 is also called 1H-Imidazole, 2-ethyl-4-methyl-. The IUPAC name is 2-ethyl-5-methyl-1H-imidazole. Its EINECS registry number is 213-234-5. In addition, the formula is C6H10N2 and the molecular weight is 110.157. It belongs to the classes of Industrial/Fine Chemicals; Imidazoles; Heterocyclic Compounds; Imidaxoles; Building Blocks; Heterocyclic Building Blocks. It is a kind of light yellow crystalline and will become clear yellow viscous liquid after melting. What's more, it should be stored in a airtight, cool and dry place.
Physical properties about this chemical are: (1)ACD/LogP: 0.62; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -1.8; (4)ACD/LogD (pH 7.4): -0.69; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 1; (8)ACD/KOC (pH 7.4): 2.5; (9)#H bond acceptors: 2; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 17.82 Å2; (13)Index of Refraction: 1.514; (14)Molar Refractivity: 33.15 cm3; (15)Molar Volume: 110 cm3; (16)Polarizability: 13.14 ×10-24cm3; (17)Surface Tension: 39.1 dyne/cm; (18)Density: 1 g/cm3; (19)Flash Point: 137.8 °C; (20)Enthalpy of Vaporization: 49.38 kJ/mol; (21)Boiling Point: 276 °C at 760 mmHg; (22)Vapour Pressure: 0.00827 mmHg at 25°C.
Preparation of 2-Ethyl-4-methylimidazole: it can be prepared by 2-ethyl-4-methylimidazoleoline. This reaction will need solvent nickel. The reaction time is 8 hours at the temperature of 180 °C by stirring. At last, you should recover nickel by filtration, vacuum distillation the filtrate and collect fraction of 150-160 °C(1.33kPa). The yield is about 70%-80%.
.gif)
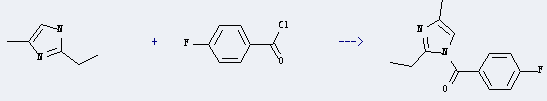
Uses of 2-Ethyl-4-methylimidazole: it is a kind of excellent temperature curing agent which can prepare epoxy glue and epoxy silicone resin coating. It has good miscibility with E-51 epoxy resin and F44 phenolic resin. There is no volatile compounds at room temperature. And it is odor, low toxicity. What's more, it has a longer period of application. It also can be used for casting, adhesive, impregnated and laminated materials. What's more, it can react with 4-fluoro-benzoyl chloride to get (2-ethyl-4-methyl-imidazol-1-yl)-(4-fluoro-phenyl)-methanone. This reaction will need reagent 1,4-diazabicyclo<2.2.2>octane(DABCO) and solvent CDCl3. The yield is about 100%.
When you are using this chemical, please be cautious about it as the following:
It is harmful if swallowed and has risk of serious damage to eyes. You should wear eye/face protection when you are using it. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: n1cc(nc1CC)C
(2)InChI: InChI=1/C6H10N2/c1-3-6-7-4-5(2)8-6/h4H,3H2,1-2H3,(H,7,8)
(3)InChIKey: ULKLGIFJWFIQFF-UHFFFAOYAC
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LDLo | intraperitoneal | 250mg/kg (250mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Veterinary and Human Toxicology. Vol. 41, Pg. 363, 1999. |
Related Products
- 2-Ethyl-4-methylimidazole
- 93138-55-7
- 93138-61-5
- 931-40-8
- 93-14-1
- 931414-11-8
- 931-44-2
- 931-50-0
- 931-51-1
- 93-15-2
- 93152-95-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View