-
Name
2-Ethylhexanol
- EINECS 203-234-3
- CAS No. 104-76-7
- Article Data177
- CAS DataBase
- Density 0.821 g/cm3
- Solubility Water Solubility :1 g/L (20 °C)
- Melting Point -76 °C(lit.)
- Formula C8H18O
- Boiling Point 184.6 °C at 760 mmHg
- Molecular Weight 130.23
- Flash Point 77.2 °C
- Transport Information
- Appearance colourless liquid
- Safety 26-36/37/39
- Risk Codes 37/38-41-36-21
-
Molecular Structure
-
Hazard Symbols
 Xn;
Xn;  Xi
Xi
- Synonyms 2-Ethyl-1-hexanol;2-Ethyl-1-hexyl alcohol;2-Ethylhexylalcohol;Conol 10WS;Ethylhexanol;G 301;NSC 9300;
- PSA 20.23000
- LogP 2.19510
Synthetic route
-
-
100528-70-9
2-(2-ethylhexyloxy)-tetrahydro-2H-pyran

-
-
104-76-7
2-Ethylhexyl alcohol

| Conditions | Yield |
|---|---|
| With methanol at 20℃; for 0.5h; | 98% |
| With methanol at 20℃; for 0.916667h; | 94% |

| Conditions | Yield |
|---|---|
| With hydrogen at 110℃; under 18751.9 Torr; for 28h; Inert atmosphere; | 96% |
| With Ni/γ-Al2O3 catalyst at 180℃; under 15001.5 Torr; for 1.33333h; Catalytic behavior; Pressure; Temperature; Time; | 93.8% |
| With sodium butanolate at 310℃; unter Druck; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; samarium diiodide; water In tetrahydrofuran for 0.00277778h; Ambient temperature; | 94% |
| With sodium hydroxide; samarium diiodide In tetrahydrofuran; water for 0.00333333h; Ambient temperature; | 94% |
| With nonan-1-al; samarium diiodide; samarium(III) trifluoromethanesulfonate In tetrahydrofuran; methanol; potassium hydroxide at 20℃; for 0.133333h; Reduction; | 93 % Chromat. |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; Raney(R) nickel 2800; isopropyl alcohol In water for 0.5h; Heating; | 90% |
| With sodium tetrahydroborate; tetrabutylammomium bromide In benzene at 20 - 25℃; for 1h; | 85% |
| With Zr(BH4)2Cl2(dabco)2 In water for 0.5h; Heating; | 85% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide; dichloro(pentamethylcyclopentadienyl) iridium; 1,7-Octadiene In para-xylene at 120℃; for 4h; Guerbet reaction; | 83% |
| With calcium carbide at 275℃; for 6h; Autoclave; | 32.6% |
| With dicarbonyl(η4-3,4-bis(4-methoxyphenyl)-2,5-diphenylcyclopenta-2,4-dienone)(iodine)ruthenium[1,3 -dimethylimidazolium]; p-benzoquinone; sodium hydroxide at 150℃; for 4h; Reagent/catalyst; Schlenk technique; Inert atmosphere; | 32.9% |

| Conditions | Yield |
|---|---|
| With Ni/γ-Al2O3 catalyst under 7500.75 Torr; | A 80.1% B 19.9% |
| With methanol; nickel boride; diborane for 0.5h; Ambient temperature; | A 51.2% B 40.9% |
| With Pd0078Co5790B42.02; hydrogen In ethanol at 99.84℃; under 7500.75 Torr; for 4h; Autoclave; | |
| With hydrogen; palladium In ethanol at 99.84℃; under 7500.75 Torr; for 4h; Autoclave; | |
| With butan-1-ol at 180℃; under 11103.3 Torr; for 4h; Reagent/catalyst; Temperature; Autoclave; Inert atmosphere; |
-
-
36653-82-4
1-Hexadecanol

-
-
5466-77-3
2-propenoic acid, 3-(4-methoxyphenyl)-, 2-ethylhexyl ester

-
A
-
104-76-7
2-Ethylhexyl alcohol

-
B
-
125628-87-7
n-hexadecyl 4-methoxycinnamate

| Conditions | Yield |
|---|---|
| With aluminium trichloride 1.) 90 deg C, 50 h, 2.) reflux, 26 h; | A n/a B 75% |

-
-
123-72-8
butyraldehyde

-
A
-
123-05-7
d,l-2-ethylhexanal

-
B
-
104-76-7
2-Ethylhexyl alcohol

-
C
-
645-62-5
2-ethylhexenal

-
D
-
71-36-3
butan-1-ol

| Conditions | Yield |
|---|---|
| With hydrogen at 180℃; under 30003 Torr; for 10h; Catalytic behavior; Autoclave; Inert atmosphere; Green chemistry; Overall yield = 97.7 %; | A 73% B 1.1% C 2% D 4.5% |
| With hydrogen at 180℃; under 30003 Torr; for 10h; Catalytic behavior; Autoclave; Inert atmosphere; Green chemistry; Overall yield = 91.7 %; | A 54.6% B 0.2% C 27.3% D 2.3% |
| With hydrogen at 180℃; under 30003 Torr; for 10h; Catalytic behavior; Autoclave; Inert atmosphere; Green chemistry; Overall yield = 100 %; | A 33% B 1.6% C 36.7% D 1.6% |
| With hydrogen; zeolite NaX; platinum at 150℃; Product distribution; further catalysts; further temperature; |

-
-
18908-66-2
2-ethylhexyl bromide

-
-
123-31-9
hydroquinone

-
A
-
104-76-7
2-Ethylhexyl alcohol

-
B
-
110126-93-7
1,4-bis((2-ethylhexyl)oxy)benzene

| Conditions | Yield |
|---|---|
| With ethanol; potassium hydroxide In methanol for 18h; Reflux; | A n/a B 64% |

-
-
123-72-8
butyraldehyde

-
A
-
104-76-7
2-Ethylhexyl alcohol

-
B
-
645-62-5
2-ethylhexenal

-
C
-
71-36-3
butan-1-ol

| Conditions | Yield |
|---|---|
| With hydrogen at 180℃; under 30003 Torr; for 10h; Catalytic behavior; Reagent/catalyst; Autoclave; Inert atmosphere; Green chemistry; Overall yield = 100 %; | A n/a B 22.8% C 58.1% |

-
-
112-72-1
1-Tetradecanol

-
-
5466-77-3
2-propenoic acid, 3-(4-methoxyphenyl)-, 2-ethylhexyl ester

-
A
-
104-76-7
2-Ethylhexyl alcohol

-
B
-
125628-86-6
n-tetradecyl 4-methoxycinnamate

| Conditions | Yield |
|---|---|
| With aluminium trichloride 1.) 90 deg C, 50 h, 2.) reflux, 24 h; | A n/a B 57.5% |

-
-
112-53-8
1-dodecyl alcohol

-
-
5466-77-3
2-propenoic acid, 3-(4-methoxyphenyl)-, 2-ethylhexyl ester

-
A
-
104-76-7
2-Ethylhexyl alcohol

-
B
-
122766-67-0
n-dodecyl 4-methoxycinnamate

| Conditions | Yield |
|---|---|
| With aluminium trichloride 1.) 90 deg C, 48 h, 2.) reflux, 24 h; | A n/a B 54% |

-
-
112-92-5
1-octadecanol

-
-
5466-77-3
2-propenoic acid, 3-(4-methoxyphenyl)-, 2-ethylhexyl ester

-
A
-
104-76-7
2-Ethylhexyl alcohol

-
B
-
42933-22-2
n-octadecyl 4-methoxycinnamate

| Conditions | Yield |
|---|---|
| With aluminium trichloride 1.) 100 deg C, 52 h, 2.) reflux, 26 h; | A n/a B 50.49% |


| Conditions | Yield |
|---|---|
| With hydrogen at 180℃; under 30003 Torr; for 10h; Catalytic behavior; Reagent/catalyst; Autoclave; Inert atmosphere; Green chemistry; Overall yield = 100 %; | A 49.4% B 39.6% |
| With hydrogen at 220℃; under 30003 Torr; for 6h; Catalytic behavior; Temperature; Pressure; |
-
-
112-72-1
1-Tetradecanol

-
-
21245-02-3
2-ethylhexyl 4-(dimethylamino)benzoate

-
A
-
104-76-7
2-Ethylhexyl alcohol

-
B
-
125628-89-9
n-tetradecyl 4-dimethylaminobenzoate

| Conditions | Yield |
|---|---|
| With aluminium trichloride 1.) heating, 58 h, 2.) reflux, 28 h; | A n/a B 48.8% |

-
-
760-67-8
2-ethylhexanoic acid chloride

-
A
-
123-05-7
d,l-2-ethylhexanal

-
B
-
104-76-7
2-Ethylhexyl alcohol

| Conditions | Yield |
|---|---|
| With tri-n-butyl-tin hydride; triphenylphosphine; indium(III) chloride In tetrahydrofuran at 20℃; for 2h; Reduction; | A 42% B 11% |
-
-
112-30-1
1-Decanol

-
-
5466-77-3
2-propenoic acid, 3-(4-methoxyphenyl)-, 2-ethylhexyl ester

-
A
-
104-76-7
2-Ethylhexyl alcohol

-
B
-
125628-85-5
n-decyl 4-methoxycinnamate

| Conditions | Yield |
|---|---|
| With aluminium trichloride 1.) 80 deg C, 24 h, 2.) reflux, 10 h; | A n/a B 35.5% |

-
-
64-17-5
ethanol

-
-
141-52-6
sodium ethanolate

-
A
-
111-87-5
octanol

-
B
-
104-76-7
2-Ethylhexyl alcohol

-
C
-
97-95-0
2-ethyl-1-butanol

-
D
-
71-36-3
butan-1-ol

-
E
-
111-27-3
hexan-1-ol

| Conditions | Yield |
|---|---|
| With [HN-(CH2CH2PiPr2)2]Mn(CO)2Br at 150℃; for 24h; Temperature; Reagent/catalyst; Guerbet Reaction; Schlenk technique; | A n/a B n/a C n/a D 33% E n/a |

-
-
112-53-8
1-dodecyl alcohol

-
-
21245-02-3
2-ethylhexyl 4-(dimethylamino)benzoate

-
A
-
104-76-7
2-Ethylhexyl alcohol

-
B
-
77016-80-9
dodecyl 4-(N,N-dimethylamino)benzoate

| Conditions | Yield |
|---|---|
| With aluminium trichloride 1.) heating, 50 h, 2.) reflux, 24 h; | A n/a B 29.37% |

-
-
112-92-5
1-octadecanol

-
-
21245-02-3
2-ethylhexyl 4-(dimethylamino)benzoate

-
A
-
104-76-7
2-Ethylhexyl alcohol

-
B
-
125628-91-3
n-octadecyl 4-dimethylaminobenzoate

| Conditions | Yield |
|---|---|
| With aluminium trichloride 1.) heating, 60 h, 2.) reflux, 30 h; | A n/a B 23.6% |

-
-
112-30-1
1-Decanol

-
-
21245-02-3
2-ethylhexyl 4-(dimethylamino)benzoate

-
A
-
104-76-7
2-Ethylhexyl alcohol

-
B
-
125628-88-8
n-decyl 4-dimethylaminobenzoate

| Conditions | Yield |
|---|---|
| With aluminium trichloride for 48h; Heating; | A n/a B 19.8% |

-
-
123-05-7
d,l-2-ethylhexanal

-
A
-
104-76-7
2-Ethylhexyl alcohol

-
B
-
7425-14-1
2-ethylhexyl 2-ethylhexanoate

| Conditions | Yield |
|---|---|
| With methanol; [carbonylchlorohydrido{bis[2-(diphenylphosphinomethyl)ethyl]amino}ethylamino] ruthenium(II); sodium methylate at 90℃; for 6h; | A 13.9% B 15.3% |
| With [carbonylchlorohydrido{bis[2-(diphenylphosphinomethyl)ethyl]amino}ethylamino] ruthenium(II); sodium methylate In methanol at 90℃; for 6h; Reagent/catalyst; | A 15.3% B 13.9% |
| With methanol; [2-(di-tert-butylphosphinomethyl)-6-(diethylaminomethyl)pyridine]ruthenium(II) chlorocarbonyl hydride; sodium methylate at 90℃; for 6h; | A 13.2% B 11.6% |

-
A
-
104-76-7
2-Ethylhexyl alcohol

-
B
-
121-33-5
vanillin

-
C
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With 1-butyl-3-methyl imidazolium tetrachloromanganate(II); ethylammonium nitrate (EAN) at 35℃; for 6h; | A n/a B 10% C n/a |

-
-
98-00-0
(2-furyl)methyl alcohol

-
-
123-72-8
butyraldehyde

-
A
-
90611-73-7
2-furfuryl-butan-1-ol

-
B
-
104-76-7
2-Ethylhexyl alcohol

| Conditions | Yield |
|---|---|
| With potassium hydroxide |


| Conditions | Yield |
|---|---|
| at 150 - 200℃; Kinetics; Hydrogenation; |

| Conditions | Yield |
|---|---|
| With aluminium; Petroleum ether nachfolgende Hydrierung, Behandeln mit Sauerstoff und Erwaermen mit wss.Salzsaeure; | |
| With zirconocene dichloride; oxygen; diisobutylaluminum chloride 1.) 40 deg C, 4 h, 2.) 4 h; Yield given. Multistep reaction; |
-
-
84612-77-1
3-ethyl-hexanoic acid, ethyl ester

-
-
104-76-7
2-Ethylhexyl alcohol

| Conditions | Yield |
|---|---|
| With ethanol; sodium |

| Conditions | Yield |
|---|---|
| With ethanol; sodium; toluene |

| Conditions | Yield |
|---|---|
| With 3,3′-(2,2-bis(hydroxymethyl)propane-1,3-diyl)bis(1-methyl-1H-imidazol-3-ium) hydrogen sulfate for 2h; Dean-Stark; Reflux; | 100% |
| Bei an einer mit einem Trockenmittel gefuellten Kolonne; |

| Conditions | Yield |
|---|---|
| With 3,3′-(2,2-bis(hydroxymethyl)propane-1,3-diyl)bis(1-methyl-1H-imidazol-3-ium) hydrogen sulfate for 2h; Dean-Stark; Reflux; | 100% |
| With diacidic ionic liquid supported on magnetic-silica nanoparticles In neat (no solvent) at 118℃; for 1h; Dean-Stark; | 100% |
| tetrabutoxytitanium for 5.41667h; azeotropic distillation; | 99.8% |

| Conditions | Yield |
|---|---|
| With diacidic ionic liquid supported on magnetic-silica nanoparticles In neat (no solvent) at 180℃; for 0.5h; Dean-Stark; | 100% |
| With toluene-4-sulfonic acid In toluene for 3h; Heating / reflux; | 97.46% |
| In 5,5-dimethyl-1,3-cyclohexadiene at 160℃; for 2h; | 97% |
| With Candida antarctica lipase B In cyclohexane at 45℃; for 24h; | 70% |
| With sulfuric acid; benzene unter Destillation des Reaktionswassers; |
-
-
93-58-3
benzoic acid methyl ester

-
-
104-76-7
2-Ethylhexyl alcohol

-
A
-
67-56-1
methanol

-
B
-
5444-75-7
Velate 368

| Conditions | Yield |
|---|---|
| With phosphorus pentoxide; potassium carbonate; Aliquat 336 In neat (no solvent) under 20 Torr; for 8h; Product distribution; Ambient temperature; | A n/a B 100% |
| With phosphorus pentoxide; potassium carbonate; Aliquat 336 In neat (no solvent) under 20 Torr; for 8h; Ambient temperature; | A n/a B 100% |

-
-
120-61-6
1,4-benzenedicarboxylic acid dimethyl ester

-
-
104-76-7
2-Ethylhexyl alcohol

-
A
-
67-56-1
methanol

-
B
-
63468-13-3
2-ethylhexyl methyl terephthalate

| Conditions | Yield |
|---|---|
| With phosphorus pentoxide; potassium carbonate; Aliquat 336 In neat (no solvent) under 20 Torr; for 24h; Product distribution; Ambient temperature; | A n/a B 100% |
| With phosphorus pentoxide; potassium carbonate; Aliquat 336 In neat (no solvent) under 20 Torr; for 24h; Ambient temperature; | A n/a B 100% |

-
-
104-76-7
2-Ethylhexyl alcohol

-
-
27247-96-7
2-ethylhexanol nitrate

| Conditions | Yield |
|---|---|
| With sulfuric acid; nitric acid at 10℃; for 0.0333333h; Large scale; | 100% |
| With sulfuric acid; nitric acid In water at 20 - 51.5℃; for 0.00111111 - 0.00222222h; Product distribution / selectivity; | 99% |
| With nitric acid; urea; europium(III) trifluoromethanesulfonate In cyclohexane at 95℃; for 10h; Catalytic behavior; Solvent; Temperature; Reagent/catalyst; Schlenk technique; | 98% |

| Conditions | Yield |
|---|---|
| at 50℃; for 120h; | 100% |
| With Novozym 435 at 80℃; under 600.048 Torr; for 144h; | |
| With Novozym435 In neat (no solvent) at 78℃; under 560 Torr; for 24h; Temperature; Enzymatic reaction; |
-
-
104-76-7
2-Ethylhexyl alcohol

-
-
10175-01-6
bis(2-methoxycarbonylethyl)tin dichloride

-
-
88261-94-3
di(2-(2-ethyl)hexoxycarbonylethyl)tin dichloride

| Conditions | Yield |
|---|---|
| In further solvent(s) addn. of Sn compd. to 2-ethyl-hexanol; | 100% |

| Conditions | Yield |
|---|---|
| With methanesulfonic acid at 20 - 85℃; for 1.5h; Inert atmosphere; | 100% |
| With toluene-4-sulfonic acid |

| Conditions | Yield |
|---|---|
| In sodium carbonate | 99.8% |
| With sodium methylate In toluene for 8h; Reflux; | 144.3 g |

| Conditions | Yield |
|---|---|
| With phenol and titanium tetraisopropoxide resin at 200℃; for 4h; Reagent/catalyst; Dean-Stark; Inert atmosphere; | 99.5% |
| With titanium(IV) isopropylate at 170 - 200℃; Inert atmosphere; Large scale; | 99% |
| With titanium(IV) isopropylate at 170 - 220℃; under 760.051 Torr; for 4.5h; Inert atmosphere; Large scale; | 99% |
-
-
104-76-7
2-Ethylhexyl alcohol

-
-
6386-38-5
methyl 3-(4-hydroxy-3,5-di-tert-butyl)phenylpropanoate

-
-
144429-84-5
2-ethylhexyl 3-(3,5-di-tert-butyl-4-hydroxy)phenylpropanoate

| Conditions | Yield |
|---|---|
| With titanium(IV) isopropylate at 160 - 170℃; for 6h; | 99.41% |
| Stage #1: 2-Ethylhexyl alcohol; methyl 3-(4-hydroxy-3,5-di-tert-butyl)phenylpropanoate In xylene for 0.5h; Heating; Stage #2: With TiO(acac)2 In xylene for 20h; Heating; | 95% |
| potassium hydroxide at 155 - 176℃; under 25 - 300 Torr; for 3.2 - 4.2h; Product distribution / selectivity; | 97.34 %Chromat. |
| potassium tert-butylate at 43 - 155℃; under 50 - 300 Torr; for 2.75h; Product distribution / selectivity; | 97.1 %Chromat. |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; N-n-butylalkylpyridinium chlorides; mixture of at 135℃; for 22h; Product distribution / selectivity; | 99.3% |
| With chloro-trimethyl-silane; dimethyl sulfoxide for 0.166667h; | 92% |
| With hydrogenchloride; alkylpyridine hydrochlorides; mixture of at 135℃; for 22 - 34h; Product distribution / selectivity; | 81.9% |
-
-
104-76-7
2-Ethylhexyl alcohol

-
-
77-92-9
citric acid

-
-
7147-34-4
tris(2-ethylhexyl)propane-1,2,3-tricarboxylate

| Conditions | Yield |
|---|---|
| With aluminum sulfate In water; Petroleum ether at 97 - 122℃; for 2.08333h; Temperature; | 99.3% |
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride at 75℃; for 4h; | 87% |

| Conditions | Yield |
|---|---|
| With [HSO3-pmim]+[HSO4]-catalyst for 0.333333h; Reagent/catalyst; Microwave irradiation; | 99.1% |
| In 5,5-dimethyl-1,3-cyclohexadiene at 160℃; for 2h; | 94% |

| Conditions | Yield |
|---|---|
| With choline chloride; zinc(II) chloride at 110℃; for 16h; | 99% |
| In 5,5-dimethyl-1,3-cyclohexadiene at 160℃; for 2h; Time; Temperature; Concentration; | 99% |
| With titanium(IV) isopropylate at 150 - 215℃; for 3.5h; Reagent/catalyst; Temperature; Large scale; | 97.23% |

| Conditions | Yield |
|---|---|
| With K5 for 0.75h; Heating; | 99% |
| With polystyrene (PS)-supported 1-(propyl-3-sulfonate) imidazolium hydrosulfate In cyclohexane at 92℃; for 3h; Fischer-Speier esterification method; water segregator; | 98.1 %Chromat. |
| In cyclohexane at 94℃; for 3h; Ionic liquid; | 98.6 %Chromat. |
-
-
104-76-7
2-Ethylhexyl alcohol

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
78016-72-5
2-ethylhexyl 4-methylbenzenesulfonate

| Conditions | Yield |
|---|---|
| With pyridine In chloroform at 0℃; Inert atmosphere; | 99% |
| With pyridine In water | |
| With pyridine In water | |
| With dmap In pyridine at 0℃; for 12h; |
-
-
104-76-7
2-Ethylhexyl alcohol

-
-
818-08-6
di(n-butyl)tin oxide

-
-
10301-02-7
1,1,3,3-tetrabutyl-1,3-bis(2-ethylhexyloxy)distannoxane

| Conditions | Yield |
|---|---|
| at 150℃; for 8h; Inert atmosphere; Industry scale; | 99% |
| at 157℃; under 150.015 - 750.075 Torr; for 2.66667h; Inert atmosphere; Large scale; | 98% |
| In toluene at 119 - 130℃; for 3.5 - 12h; Product distribution / selectivity; Heating / reflux; | 95% |
| at 150℃; under 760.051 Torr; for 8.5h; Inert atmosphere; Industry scale; | 99 %Spectr. |

| Conditions | Yield |
|---|---|
| With pseudomonas fuorescens lipase immobilized on multiwall carbon nano-tubes at 50℃; for 5h; Green chemistry; | 99% |
| With steapsin lipase In hexane at 55℃; for 30h; Enzymatic reaction; | 99 %Chromat. |
-
-
104-76-7
2-Ethylhexyl alcohol

-
-
10375-34-5
2,5-furandicarbonyl dichloride

-
-
158099-01-5
2,5-furandicarboxylic acid di(2-ethylhexyl) ester

| Conditions | Yield |
|---|---|
| at 80 - 100℃; Inert atmosphere; | 99% |
| at 75℃; for 4h; | 85% |

| Conditions | Yield |
|---|---|
| With titanium(IV) isopropylate at 170 - 220℃; for 4.5h; Large scale; | 99% |
| In 5,5-dimethyl-1,3-cyclohexadiene at 160℃; for 2h; | 51% |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In toluene at 120℃; for 24h; Inert atmosphere; | 99% |
-
-
104-76-7
2-Ethylhexyl alcohol

-
-
18908-66-2
2-ethylhexyl bromide

| Conditions | Yield |
|---|---|
| With hydrogen bromide; Aliquat 336 In chlorobenzene at 100℃; for 14h; without cat.; | 98.8% |
| With phosphorus tribromide at 20 - 65℃; Large scale; | 96.6% |
| With tribromo-isocyanuric acid; triphenylphosphine In dichloromethane at 20℃; for 1.5h; | 67% |
-
-
104-76-7
2-Ethylhexyl alcohol

-
-
27899-21-4
2,4-diethylglutaric acid

-
-
499195-64-1
bis(2-ethylhexyl) 2,4-diethylglutarate

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene for 5h; Heating / reflux; | 98.4% |
| With toluene-4-sulfonic acid In toluene for 5h; Heating / reflux; | 98.4% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In tetrahydrofuran at 5 - 20℃; for 3.33333h; | 98.3% |
-
-
104-76-7
2-Ethylhexyl alcohol

| Conditions | Yield |
|---|---|
| With dmap; oxo[hexa(trifluoroacetato)]tetrazinc In toluene at 110℃; Inert atmosphere; Molecular sieve; | 98.3% |

| Conditions | Yield |
|---|---|
| Stage #1: maleic anhydride; 2-Ethylhexyl alcohol at 60 - 130℃; under 760.051 Torr; for 3.5h; Stage #2: With sodium hydrogensulfite; sodium hydroxide In water at 107℃; for 1h; Reagent/catalyst; Temperature; | 98.2% |

| Conditions | Yield |
|---|---|
| With titanium(IV) isopropylate In water at 170 - 220℃; for 4.5h; Inert atmosphere; | 98% |
| With hypophosphorous acid; toluene-4-sulfonic acid at 140℃; for 12h; | 92% |
| With toluene-4-sulfonic acid; benzene |
2-Ethylhexanol Specification
The CAS registry number of 2-Ethylhexanol is 104-76-7. Its EINECS registry number is 203-234-3. The IUPAC name is 2-ethylhexan-1-ol. In addition, the molecular formula is C8H18O and the molecular weight is 130.23. It is also called 1-hexanol, 2-ethyl-. What's more, it is a kind of colourless liquid that is nearly insoluble in water but soluble in most organic solvents. And it belongs to the class of Industrial/Fine Chemicals. Besides, it should be stored in sealed container, and put in a cool and dry place. The storage place must stay away from oxidant, the fire, water source and heat source
Physical properties about this chemical are: (1)ACD/LogP: 2.82; (2)ACD/LogD (pH 5.5): 2.82; (3)ACD/LogD (pH 7.4): 2.82; (4)ACD/BCF (pH 5.5): 81.41; (5)ACD/BCF (pH 7.4): 81.41; (6)ACD/KOC (pH 5.5): 811.45; (7)ACD/KOC (pH 7.4): 811.45; (8)#H bond acceptors: 1; (9)#H bond donors: 1; (10)#Freely Rotating Bonds: 6; (11)Polar Surface Area: 9.23 Å2; (12)Index of Refraction: 1.426; (13)Molar Refractivity: 40.6 cm3; (14)Molar Volume: 158.4 cm3; (15)Polarizability: 16.09 ×10-24cm3; (16)Surface Tension: 28 dyne/cm; (17)Density: 0.821 g/cm3; (18)Flash Point: 77.2 °C; (19)Enthalpy of Vaporization: 48.98 kJ/mol; (20)Boiling Point: 184.6 °C at 760 mmHg; (21)Vapour Pressure: 0.207 mmHg at 25°C.
Preparation of 2-Ethylhexanol: it is produced industrially by the aldol condensation of n-butyraldehyde, followed by hydrogenation of the resulting hydroxyaldehyde. The n-butyraldehyde is made by hydroformylation of propylene, either in a self-contained plant or as the first step in a fully-integrated facility. The equation is as follows:
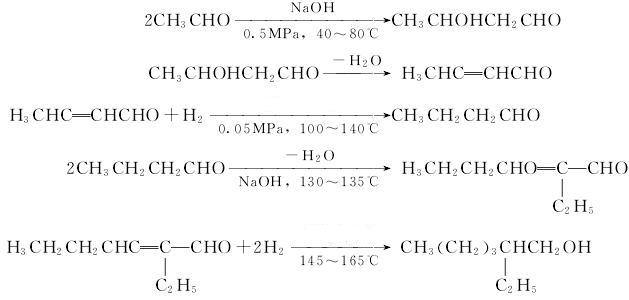
Uses of 2-Ethylhexanol: It is commonly used as a low volatility solvent. And it is used in the production of plasticizers, defoaming agents, dispersants, antioxidants and oil additives. In addition, it can be used to get 3-bromomethyl-heptane. This reaction will need reagent 48percent aq. HBr, catalyst aliquat 336 and solvent chlorobenzene. The reaction time is 14 hours at reaction temperature of 100 °C. The yield is about 98.8%.

When you are using this chemical, please be cautious about it as the following:
This chemical is irritating respiratory system. And it is harmful in contact with skin. Moreover, it has risk of serious damage to eyes. When you are using it, wear suitable protective clothing, gloves and eye/face protection. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: OCC(CC)CCCC
(2)InChI: InChI=1/C8H18O/c1-3-5-6-8(4-2)7-9/h8-9H,3-7H2,1-2H3
(3)InChIKey: YIWUKEYIRIRTPP-UHFFFAOYAU
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LD50 | oral | 1860mg/kg (1860mg/kg) | Zeitschrift fuer die Gesamte Hygiene und Ihre Grenzgebiete. Vol. 20, Pg. 575, 1974. | |
| mouse | LD50 | intraperitoneal | 759mg/kg (759mg/kg) | Zeitschrift fuer die Gesamte Hygiene und Ihre Grenzgebiete. Vol. 20, Pg. 575, 1974. | |
| mouse | LD50 | oral | 2500mg/kg (2500mg/kg) | Zeitschrift fuer die Gesamte Hygiene und Ihre Grenzgebiete. Vol. 20, Pg. 575, 1974. | |
| mouse | LD50 | parenteral | 1670mg/kg (1670mg/kg) | Food and Cosmetics Toxicology. Vol. 17, Pg. 775, 1979. | |
| rabbit | LD50 | oral | 1180mg/kg (1180mg/kg) | Zeitschrift fuer die Gesamte Hygiene und Ihre Grenzgebiete. Vol. 20, Pg. 575, 1974. | |
| rabbit | LD50 | skin | 1970mg/kg (1970mg/kg) | Raw Material Data Handbook, Vol.1: Organic Solvents, 1974. Vol. 1, Pg. 61, 1974. | |
| rat | LC | inhalation | > 2000ppm/6H (2000ppm) | Kodak Company Reports. Vol. 21MAY1971, | |
| rat | LD50 | intraperitoneal | 500mg/kg (500mg/kg) | Hydrometallurgy. Vol. 3, Pg. 201, 1978. | |
| rat | LD50 | oral | 3730mg/kg (3730mg/kg) | BRAIN AND COVERINGS: RECORDINGS FROM SPECIFIC AREAS OF CNS BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LUNGS, THORAX, OR RESPIRATION: DYSPNEA | American Industrial Hygiene Association Journal. Vol. 34, Pg. 493, 1973. |
| rat | LD50 | parenteral | 4600mg/kg (4600mg/kg) | Food and Cosmetics Toxicology. Vol. 17, Pg. 775, 1979. | |
| rat | LD50 | skin | > 3gm/kg (3000mg/kg) | National Technical Information Service. Vol. OTS0524344, | |
| rat | LD50 | subcutaneous | 650mg/kg (650mg/kg) | Raw Material Data Handbook, Vol.1: Organic Solvents, 1974. Vol. 1, Pg. 61, 1974. |
Related Products
- 2-Ethylhexanol
- 2-Ethylhexanol distillation residuum
- 10476-81-0
- 10476-85-4
- 10476-86-5
- 10476-95-6
- 104771-35-9
- 1047724-24-2
- 104774-87-0
- 104774-88-1
- 104774-94-9
- 104775-30-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View