-
Name
2-Methyl-1,3-cyclopentanedione
- EINECS 212-153-2
- CAS No. 765-69-5
- Article Data49
- CAS DataBase
- Density 1.102 g/cm3
- Solubility
- Melting Point 212-215 °C(lit.)
- Formula C6H8O2
- Boiling Point 218 °C at 760 mmHg
- Molecular Weight 112.128
- Flash Point 78.5 °C
- Transport Information
- Appearance light beige to tan crystalline powder
- Safety 22-24/25-36-26
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 2-Methyl-1,3-cyclopentadione;NSC 54458;
- PSA 34.14000
- LogP 0.55450
Synthetic route

| Conditions | Yield |
|---|---|
| In methanol Irradiation (UV/VIS); anaerobic irradiation 1h with visible light at 20°C; analyzing products by GLC; | A 69% B 19% |
| In water Irradiation (UV/VIS); anaerobic irradiation 1h with visible light in single-compartment vesicle of N,N-dihexadecyl-Nα-(6-(trimethylammonio)hexanoyl)-L-alaninamide bromide at 20°C; phosphate-borate buffer; analyzing products by GLC; | A 19% B 67% |
| In benzene Irradiation (UV/VIS); anaerobic irradiation 1h with visible light at 20°C; analyzing products by GLC; | A 57% B 28% |
-
-
614-45-9
tert-Butyl peroxybenzoate

-
-
3859-41-4
1,3-cyclopentadione

-
-
765-69-5
2-Methylcyclopentane-1,3-dione

| Conditions | Yield |
|---|---|
| With acetic acid; copper(l) chloride at 120℃; | 68% |

| Conditions | Yield |
|---|---|
| Stage #1: succinic acid With aluminum (III) chloride; nitromethane at 20℃; for 1h; Inert atmosphere; Stage #2: propionyl chloride at 80℃; for 3h; Inert atmosphere; | 63% |
| With aluminum oxide 1.) nitromethane, 2.) 80 deg C, 3 h; Multistep reaction; | |
| Stage #1: succinic acid With aluminum (III) chloride In nitromethane for 3.5h; Reflux; Inert atmosphere; Stage #2: propionyl chloride In nitromethane for 2.5h; Reflux; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With semicarbazide hydrochloride anschliessendes Erhitzen mit KOH in Aethylenglykol; |
-
-
4505-54-8
3-methylcyclopentane-1,2,4-trione

-
A
-
765-69-5
2-Methylcyclopentane-1,3-dione

-
B
-
4800-04-8
4-hydroxy-2-methyl-1,3-cyclopentanedione

| Conditions | Yield |
|---|---|
| With ethanol; platinum Hydrogenation; | |
| With ethanol; platinum |

| Conditions | Yield |
|---|---|
| With aluminium trichloride; nitromethane 1.) heating, 2.) 80 deg C, 3 h; Yield given. Multistep reaction; |
-
-
125113-32-8
2-(1-ethoxy-ethyl)-2-trimethylsilanyloxy-cyclobutanone

-
-
765-69-5
2-Methylcyclopentane-1,3-dione

| Conditions | Yield |
|---|---|
| With Nafion-H; trifluoroacetic acid at 85℃; for 10h; Yield given; |

| Conditions | Yield |
|---|---|
| With 9,10-dihydroanthracene In 1,3,5-trimethyl-benzene at 221℃; Kinetics; Rate constant; different temperatures; ΔG(excit.)300, ΔH(excit.), ΔS(excit.); | A n/a B 75 % Chromat. |

| Conditions | Yield |
|---|---|
| With 9,10-dihydroanthracene In 1,3,5-trimethyl-benzene at 221℃; Kinetics; Rate constant; different temperatures; ΔG(excit.)300, ΔH(excit.), ΔS(excit.); | A n/a B 82 % Chromat. |

| Conditions | Yield |
|---|---|
| N+C5Ala2C16 vesicle at 20℃; Irradiation; in aqueous phosphate-borate buffer; | A 19 % Chromat. B 67 % Chromat. |
| In methanol at 20℃; Irradiation; | A 69 % Chromat. B 19 % Chromat. |
-
-
17082-61-0
1,2-bis(trimethylsiloxy)cyclobutene

-
-
534-15-6
1,1-dimethoxyethane

-
-
765-69-5
2-Methylcyclopentane-1,3-dione

| Conditions | Yield |
|---|---|
| Stage #1: 1,2-bis(trimethylsiloxy)cyclobutene; 1,1-dimethoxyethane With boron trifluoride diethyl etherate In dichloromethane at -78 - 20℃; for 4h; Stage #2: With trifluoroacetic acid for 24h; Heating; Further stages.; |
-
-
781-38-4
(3-methyl-2,4,5-trioxo-cyclopentyl)-glyoxylic acid ethyl ester

-
-
765-69-5
2-Methylcyclopentane-1,3-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: aqueous phosphoric acid 2: water containing ethanol; platinum View Scheme |
-
-
393125-77-4
C6H10N2O2

-
-
765-69-5
2-Methylcyclopentane-1,3-dione

| Conditions | Yield |
|---|---|
| With gold(III) tribromide; dimethylglyoxal In ethanol; water at 20℃; for 15h; pH=7; | 100 %Spectr. |

| Conditions | Yield |
|---|---|
| With diethyl 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate; rac-Pro-OH In ethanol at 25℃; |
-
-
765-69-5
2-Methylcyclopentane-1,3-dione

-
-
78-83-1
2-methyl-propan-1-ol

-
-
22117-97-1
3-isobutoxy-2-methyl-2-cyclopenten-1-one

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In benzene for 16h; Heating; | 100% |
| With toluene-4-sulfonic acid | 100% |
| With toluene-4-sulfonic acid In benzene for 8h; Heating; | 99% |
-
-
765-69-5
2-Methylcyclopentane-1,3-dione

-
-
78-94-4
methyl vinyl ketone

-
-
25112-78-1
2-methyl-2-(3-oxobutyl)-1,3-cyclopentanedione

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 20℃; | 100% |
| With acetic acid In water at 75℃; for 4h; | 98% |
| With triethylamine In dichloromethane for 12h; | 95% |
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
765-69-5
2-Methylcyclopentane-1,3-dione

-
-
3883-56-5
3-methoxy-2-methylcyclopent-2-enone

| Conditions | Yield |
|---|---|
| In diethyl ether | 100% |
-
-
765-69-5
2-Methylcyclopentane-1,3-dione

-
-
1629-58-9
4-penten-3-one

-
-
34927-37-2
2-(3-oxopentyl)-2-methylcyclopentane-1,3-diones

| Conditions | Yield |
|---|---|
| With acetic acid; hydroquinone In water at 75℃; | 100% |
| With triethylamine In ethyl acetate for 120h; | 90% |
| With triethylamine In ethyl acetate at 25℃; for 12h; | 89% |
-
-
765-69-5
2-Methylcyclopentane-1,3-dione

-
-
107-02-8
acrolein

-
-
66976-99-6
2-methyl-2-(2-formylethyl)-1,3-cyclopentanedione

| Conditions | Yield |
|---|---|
| In water Ambient temperature; | 100% |
| In water at 20℃; for 18h; | 100% |
| In water at 20℃; for 18h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran | 100% |
-
-
765-69-5
2-Methylcyclopentane-1,3-dione

-
-
77-78-1
dimethyl sulfate

-
-
3883-56-5
3-methoxy-2-methylcyclopent-2-enone

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 8h; Reflux; | 99% |
| With potassium carbonate In acetone at 20 - 60℃; for 8h; | 95% |
| With potassium carbonate In acetone at 20℃; for 4h; Reflux; |
-
-
765-69-5
2-Methylcyclopentane-1,3-dione

-
-
31706-95-3
1'-Hydroxymethyleugenol

-
-
160514-96-5
2-(3',4'-dimethoxycinnamyl)-2-methylcyclopentane-1,3-dione

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran for 0.5h; Product distribution; Heating; further solvents and catalysts; | 98.6% |
| With tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran for 0.5h; Heating; | 98.6% |
-
-
765-69-5
2-Methylcyclopentane-1,3-dione

-
-
999-97-3
1,1,1,3,3,3-hexamethyl-disilazane

-
-
84348-14-1
2-methyl-3-(trimethylsilyloxy)cyclopent-2-enone

| Conditions | Yield |
|---|---|
| With 1H-imidazole at 120℃; for 2h; Neat (no solvent); | 98% |
| With 1H-imidazole Heating; | |
| With 1H-imidazole at 20 - 130℃; for 3h; Inert atmosphere; |
-
-
765-69-5
2-Methylcyclopentane-1,3-dione

-
-
107-18-6
allyl alcohol

-
-
26828-48-8
2-allyl-2-methyl-1,3-cyclopentanedione

| Conditions | Yield |
|---|---|
| With Ru(Cp*)(η3-C3H5)(p-CH3C6H5SO3)2 In dichloromethane; acetonitrile at 50℃; for 3h; regioselective reaction; | 98% |
| With tetrakis(triphenylphosphine) palladium(0); carbon dioxide In dichloromethane under 15200 Torr; for 24h; Ambient temperature; | 65% |
-
-
765-69-5
2-Methylcyclopentane-1,3-dione

-
-
623-05-2
(4-hydroxyphenyl)methanol

-
-
112621-27-9
2-(4-hydroxybenzyl)-2-methyl-1,3-cyclopentanedione

| Conditions | Yield |
|---|---|
| In water at 80℃; for 12h; | 97% |
-
-
765-69-5
2-Methylcyclopentane-1,3-dione

-
-
57-14-7
1,1-dimethylhydrazine

-
-
124015-86-7
2-methyl-1,3-cyclopentanedione-dimethylhydrazone

| Conditions | Yield |
|---|---|
| for 4h; Heating; | 97% |
| With toluene-4-sulfonic acid In benzene Heating; | 96% |
-
-
765-69-5
2-Methylcyclopentane-1,3-dione

-
-
86422-21-1
(E)-3-(trimethylsilyl)-2-propenyl acetate

-
-
147974-05-8
2-Methyl-2-((E)-3-trimethylsilanyl-allyl)-cyclopentane-1,3-dione

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene; tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran for 34h; Ambient temperature; | 96% |
-
-
765-69-5
2-Methylcyclopentane-1,3-dione

-
-
56778-49-5
3-iodo-2-methyl-2-cyclopentenone

| Conditions | Yield |
|---|---|
| With iodine; triethylamine; triphenylphosphine In acetonitrile at 80 - 90℃; for 6h; | 96% |
| With triphenylphosphine diiodide | 92% |
| With triphenylphosphine diiodide; triethylamine In acetonitrile for 3h; Heating; | 92% |
-
-
765-69-5
2-Methylcyclopentane-1,3-dione

-
-
51410-44-7
1-(4-methoxylphenyl)-2-propen-1-ol

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran Heating; | 95.4% |
-
-
765-69-5
2-Methylcyclopentane-1,3-dione

-
-
78-94-4
methyl vinyl ketone

-
-
19576-08-0
(+/-)-7a-methyl-2,3,7,7a,tetrahydro-1H-indene-1,5(6H)-dione

| Conditions | Yield |
|---|---|
| With lipase from porcine pancreas In methanol; water Robinson Annulation; Green chemistry; Enzymatic reaction; | 95% |
| Stage #1: 2-Methylcyclopentane-1,3-dione; methyl vinyl ketone With aluminum oxide at 20℃; for 0.166667h; Michael addition; Stage #2: With pyrrolidine for 0.05h; Robinson annulation; microwave irradiation; | 45% |
| With pyridine; toluene; tert-butyl alcohol Erhitzen des Reaktionsprodukts mit Essigsaeure und Toluol-4-sulfonsaeure; | |
| With triethylamine In ethyl acetate |
-
-
765-69-5
2-Methylcyclopentane-1,3-dione

-
-
78500-02-4
4,10-bisthia-1,2,3,4,9,10-hexahydrophenanthrenylideneethylisothiuroniumacetate

-
-
78500-03-5
8,14-seco-1,6-dithiabenz<3,4>estra-3,5(10),9(11)-triene-14,17-dione

| Conditions | Yield |
|---|---|
| In diethyl ether; water Ambient temperature; two phase system; | 95% |
| In diethyl ether; water for 6h; | 95% |
-
-
765-69-5
2-Methylcyclopentane-1,3-dione

| Conditions | Yield |
|---|---|
| In benzene at 25℃; for 48h; | 95% |
-
-
765-69-5
2-Methylcyclopentane-1,3-dione

-
-
358-23-6
trifluoromethylsulfonic anhydride

-
-
134050-29-6
trifluoromethanesulfonic acid 2-methyl-3-oxocyclopent-1-enyl ester

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at -78 - -40℃; for 1h; | 95% |
| With pyridine In dichloromethane at -78 - 20℃; Inert atmosphere; | 92% |
| Stage #1: 2-Methylcyclopentane-1,3-dione With N-ethyl-N,N-diisopropylamine In dichloromethane at -78℃; for 0.166667h; Sealed tube; Inert atmosphere; Stage #2: trifluoromethylsulfonic anhydride In dichloromethane at -78℃; for 1h; Sealed tube; Inert atmosphere; | 92% |
| With 2,6-dimethylpyridine In dichloromethane at -78 - 20℃; for 1.5h; |
-
-
765-69-5
2-Methylcyclopentane-1,3-dione

-
-
627-27-0
homoalylic alcohol

-
-
1116036-47-5
3-but-3-enyloxy-2-methyl-cyclopent-2-enone

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene for 12h; Reflux; | 95% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid for 14h; Diels-Alder Cycloaddition; Reflux; | 95% |


| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 30℃; for 0.5h; Reagent/catalyst; Solvent; Temperature; Inert atmosphere; | 95% |
-
-
765-69-5
2-Methylcyclopentane-1,3-dione

-
-
35173-23-0
3-chloro-2-methylcyclopent-2-en-1-one

| Conditions | Yield |
|---|---|
| With oxalyl dichloride; N,N-dimethyl-formamide In dichloromethane 0 deg C, 5 min, then to room temp., 30 min; | 94% |
| With triethylamine; dichlorotriphenylphosphorane In benzene for 4h; Ambient temperature; | 92% |
| With lithium hydride; O-phenyl phosphorodichloridate; lithium chloride In tetrahydrofuran for 2h; Ambient temperature; | 83% |
-
-
765-69-5
2-Methylcyclopentane-1,3-dione

-
-
591-87-7
Allyl acetate

-
-
26828-48-8
2-allyl-2-methyl-1,3-cyclopentanedione

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0) In methanol Heating; | 94% |
| tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran at 25℃; | 94% |
| tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran for 72h; | 76% |
-
-
765-69-5
2-Methylcyclopentane-1,3-dione

-
-
131943-93-6
6-(2-benzyloxy-3-methoxyphenyl)-1-hexen-3-one

-
-
131943-94-7
(+/-)-2-<6-(2-benzyloxy-3-methoxyphenyl)-3-oxohexyl>-2-methylcyclopentane-1,3-dione

| Conditions | Yield |
|---|---|
| With triethylamine In ethyl acetate for 36h; Ambient temperature; | 94% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-Methylcyclopentane-1,3-dione With magnesium(II) perchlorate at 0 - 5℃; for 0.0833333h; Stage #2: With N-bromosaccharin at 0 - 5℃; | 94% |
| With bromine; triethylamine In ethyl acetate at 80℃; for 1h; | |
| With methanol; tetraethylammonium bromide; Dess-Martin periodane In chloroform at 20℃; for 0.0833333h; |
-
-
765-69-5
2-Methylcyclopentane-1,3-dione

-
-
463-49-0
1,2-propanediene

-
-
115375-59-2
6-methoxy-3,4-dihydronaphthalene-1-yl triflate

-
A
-
57346-12-0
2-<2-(6-methoxy-3,4-dihydronaphthalen-1-yl)prop-2-ene-1-yl>-2-methylcyclopentane-1,3-dione

-
C
-
183194-89-0
2-Methyl-2-(3-methyl-2-methylene-but-3-enyl)-cyclopentane-1,3-dione

| Conditions | Yield |
|---|---|
| With sodium hydride; triphenylphosphine; lithium chloride; bis(dibenzylideneacetone)-palladium(0) In dimethyl sulfoxide at 65℃; for 76h; | A 94% B 5% C 16% |

-
-
765-69-5
2-Methylcyclopentane-1,3-dione

-
-
74-88-4
methyl iodide

-
-
3883-58-7
2,2-dimethylcyclopentane-1,3-dione

| Conditions | Yield |
|---|---|
| Stage #1: 2-Methylcyclopentane-1,3-dione; methyl iodide With potassium hydroxide In 1,4-dioxane; water Heating / reflux; Stage #2: With hydrogenchloride In water at 120℃; for 0.25h; | 93% |
| With potassium hydroxide In 1,4-dioxane; water at 20℃; Reflux; | 93% |
| With potassium hydroxide In 1,4-dioxane; water Reflux; | 68% |
-
-
530-48-3
1,1-Diphenylethylene

-
-
765-69-5
2-Methylcyclopentane-1,3-dione

-
A
-
119-61-9
benzophenone

-
B
-
142605-75-2
1-hydroxy-6-methyl-4,4-diphenyl-2,3-dioxabicyclo<4.3.0>nonan-7-one

| Conditions | Yield |
|---|---|
| With oxygen; manganese(II) acetate In acetic acid at 23℃; for 15h; | A 5% B 93% |

-
-
765-69-5
2-Methylcyclopentane-1,3-dione

-
-
56671-87-5
3-Bromo-2-methylcyclopent-2-en-1-one

| Conditions | Yield |
|---|---|
| With triethylamine; dibromotriphenylphosphorane In benzene for 4h; Ambient temperature; | 93% |
| With Oxalyl bromide; N,N-dimethyl-formamide In dichloromethane 0 deg C, 5 min, then to room temp., 30 min; | 88% |
| With Oxalyl bromide; N,N-dimethyl-formamide In dichloromethane at 0 - 20℃; for 4.25h; Inert atmosphere; | 83% |
2-Methyl-1,3-cyclopentanedione Chemical Properties
The Molecular Weight of 2-Methyl-1,3-cyclopentanedione(765-69-5):112.13
The Molecular Structure of 2-Methyl-1,3-cyclopentanedione(765-69-5) is:
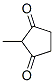
Density:1.102 g/cm3
Melting point:212-215 °C(lit.)
Boiling point:218 °C at 760 mmHg
Flash point:78.5 °C
Index of Refraction:1.463
Molar Refractivity:28.02 cm3
Molar Volume:101.7 cm3
Polarizability:11.11 10-24m3
Surface Tension:35.9 dyne/cm
Enthalpy of Vaporization:45.44 kJ/mol
Vapour Pressure:0.129 mmHg at 25°C
Appearance:light beige - tan and crystalline powder
IUPAC Name:2-methylcyclopentane-1,3-dione
Synonyms:2-Methyl-1,3-cyclopentadione;2-METHYLCYCLOPENTAN-1,3-DIONE;2-METHYL-1,3-CYCLOPENTANEDIONE;METHYLCYCLE-D;2-Methylcyclopentane-1,3-dione,98%;2-METHYL-1,3-CYCLOPENTANEDIONE(METHYLCYCLO-D);1,3-Cyclopentanedione, 2-methyl-
2-Methyl-1,3-cyclopentanedione Uses
2-Methyl-1,3-cyclopentanedione Toxicity Data With Reference
LD50/LC50: RTECS: Not available.
Carcinogenicity: 2-Methyl-1,3-cyclopentanedione - Not listed as a carcinogen by ACGIH, IARC, NTP, or CA Prop 65.
2-Methyl-1,3-cyclopentanedione Safety Profile
Hazard Codes
Xi:Irritant
Risk Statements
R36/37/38:Irritating to eyes, respiratory system and skin .
Safety Statements
S22:Do not breathe dust .
S24/25:Avoid contact with skin and eyes .
S36:Wear suitable protective clothing .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
WGK Germany:3
Hazard Note:rritant
HS Code:9142900
2-Methyl-1,3-cyclopentanedione Specification
Chemical Stability: Stable under normal temperatures and pressures.
Hazardous Decomposition Products Carbon monoxide, carbon dioxide.
Hazardous Polymerization Will not occur.
Conditions to Avoid: Incompatible materials.
Incompatibilities with Other Materials Strong oxidizing agents.
Related Products
- 2-METHYL-1-((2-METHYLCYCLOHEXYL)-CARBONYL)PIPERIDINE
- 2-METHYL-1-((6-METHYL-3-CYCLOHEXEN-1-YL)CARBONYL)PIPERIDINE
- 2-Methyl-1-(naphthalen-2-yl)propan-2-amine
- 2-Methyl-1-(phenylsulfonyl)-1H-pyrrolo[2,3-b]pyridine
- 2-Methyl-1,10-phenanthroline
- 2-METHYL-1,2,3,4-TETRAHYDROQUINOLINE-5-METHANOL
- 2-METHYL-1,2,3,6-TETRAHYDROBENZ-ALDEHYDE
- 2-Methyl-1,2-benzothiazol-3(2H)-one
- 2-METHYL-1,2-DI-3-PYRIDYL-1-PROPANONE TARTRATE (1:2)
- 2-Methyl-1,2-oxaphospholan-5-one 2-oxide
- 765-70-8
- 7657-09-2
- 76574-43-1
- 76575-71-8
- 76576-67-5
- 76578-12-6
- 76578-14-8
- 76578-90-0
- 76579-64-1
- 7658-08-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View