-
Name
2-Methyl-3-biphenylmethanol
- EINECS 1312995-182-4
- CAS No. 76350-90-8
- Article Data25
- CAS DataBase
- Density 1.073 g/cm3
- Solubility Insoluble in water, soluble in ethanol, benzene and toluene
- Melting Point 73-76 °C(lit.)
- Formula C14H14O
- Boiling Point 330.922 °C at 760 mmHg
- Molecular Weight 198.265
- Flash Point 143.013 °C
- Transport Information
- Appearance white to light yellow crystal powder
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 2-Methyl-3-phenylbenzylalcohol;2-Methylbiphenyl-3-ylmethanol;3-Hydroxymethyl-2-methylbiphenyl;2-Methyl-3-biphenylmethanol;
- PSA 20.23000
- LogP 3.15430
Synthetic route
-
-
83647-43-2
3-bromo-2-methylbenzyl alcohol

-
-
98-80-6
phenylboronic acid

-
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

| Conditions | Yield |
|---|---|
| With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; sodium hydrogencarbonate In ethanol; toluene at 80℃; for 0.5h; Suzuki-Miyaura Coupling; Inert atmosphere; | 98% |
| With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; cesium acetate In tetrahydrofuran Suzuki-Miyaura Coupling; Reflux; | 98% |
| With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; sodium hydrogencarbonate In ethanol; toluene at 80℃; for 3h; Inert atmosphere; | 98% |
-
-
502483-86-5
1,3-dihydro-4-phenylisobenzofuran

-
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

| Conditions | Yield |
|---|---|
| Stage #1: 1,3-dihydro-4-phenylisobenzofuran With lithium; 4,4'-di-tert-butylbiphenyl In tetrahydrofuran at -78℃; for 3h; Stage #2: With water In tetrahydrofuran at -78℃; for 0.333333h; Stage #3: With water In tetrahydrofuran at -78 - 20℃; Further stages.; | 95% |
| Multi-step reaction with 2 steps 1.1: Li; DTBB / tetrahydrofuran / 3 h / -78 °C 2.1: H2O / 0.5 h / -78 °C 2.2: H2O / -78 - 20 °C View Scheme |
-
-
84541-46-8
3-chloromethyl-2-methyl-[1,1'-biphenyl]

-
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; water; sodium acetate at 100℃; for 15h; Reagent/catalyst; | 91% |

-
A
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

-
B
-
76023-99-9
(1R,3R)-3-((Z)-2-chloro-3,3,3-trifluoroprop-1-en-1-yl)-2,2,-dimethylcyclopropane-1-carboxylic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol for 2h; Hydrolysis; Heating; |

-
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

| Conditions | Yield |
|---|---|
| Stage #1: C14H12OLi(1-)*Li(1+) With water at -78℃; for 0.5h; Stage #2: With water at -78 - 20℃; Further stages.; |

-
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: palladium 2.1: Li; DTBB / tetrahydrofuran / 3 h / -78 °C 3.1: H2O / 0.5 h / -78 °C 3.2: H2O / -78 - 20 °C View Scheme |
-
-
576-23-8
2,3-dimethylbromobenzene

-
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: N-bromosuccinimide; AIBN 2.1: tetrabutylammonium hydroxide / dioxane 3.1: palladium 4.1: Li; DTBB / tetrahydrofuran / 3 h / -78 °C 5.1: H2O / 0.5 h / -78 °C 5.2: H2O / -78 - 20 °C View Scheme |
-
-
127168-82-5
1-bromo-2,3-bis(bromomethyl)benzene

-
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: tetrabutylammonium hydroxide / dioxane 2.1: palladium 3.1: Li; DTBB / tetrahydrofuran / 3 h / -78 °C 4.1: H2O / 0.5 h / -78 °C 4.2: H2O / -78 - 20 °C View Scheme |

-
A
-
74609-46-4
3-(2-chloro-3,3,3-trifluoroprop-1-en-yl)-2,2-dimethylcyclopropanecarboxylic acid

-
B
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

| Conditions | Yield |
|---|---|
| With carboxylesterase EstSt7 from Sulfolobus tokodaii strain 7; water In ethanol at 80℃; pH=9; Kinetics; Enzymatic reaction; |
-
-
76006-33-2
3-bromo-2-methylbenzoic acid

-
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: borane-THF / tetrahydrofuran / 20 °C / Cooling with ice 2: dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; sodium hydrogencarbonate / toluene; ethanol / 0.5 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 2 steps 1: borane-THF / tetrahydrofuran / 15 h / 0 - 20 °C / Inert atmosphere 2: dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; sodium hydrogencarbonate / ethanol; toluene / 0.5 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 2 steps 1: borane-THF / tetrahydrofuran / 15 h / 0 - 20 °C / Inert atmosphere 2: dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; sodium hydrogencarbonate / toluene; ethanol / 0.5 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1.1: sulfuric acid / 60 °C 2.1: lithium aluminium tetrahydride / tetrahydrofuran / 2 h / 0 - 20 °C 2.2: 1 h / 0 °C 3.1: potassium carbonate; tetrakis(triphenylphosphine) palladium(0) / 1,4-dioxane; water / 3 h / 100 °C / Inert atmosphere View Scheme |
-
-
89951-60-0
2-methyl-[1,1'-biphenyl]-3-carbaldehyde

-
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

| Conditions | Yield |
|---|---|
| With hydrogen In isopropyl alcohol; toluene at 80℃; under 9000.9 Torr; for 2h; Solvent; Temperature; Reagent/catalyst; Pressure; Autoclave; |
-
-
99548-54-6
methyl 3-bromo-2-methylbenzoate

-
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: lithium aluminium tetrahydride / tetrahydrofuran / 2 h / 0 - 20 °C 1.2: 1 h / 0 °C 2.1: potassium carbonate; tetrakis(triphenylphosphine) palladium(0) / 1,4-dioxane; water / 3 h / 100 °C / Inert atmosphere View Scheme |
-
-
78246-90-9
cyhalothrin chloride

-
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

-
-
82657-04-3
rel-(1R,3R)-(2-methyl[1,1'-biphenyl]-3-yl)methyl 3-[(1Z)-2-chloro-3,3,3-trifluoro-1-propenyl]-2,2-dimethylcyclopropanecarboxylate

| Conditions | Yield |
|---|---|
| With hexamethylenetetramine at 40 - 45℃; for 5h; Temperature; Reagent/catalyst; Large scale; | 98.5% |
-
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

-
-
84541-46-8
3-chloromethyl-2-methyl-[1,1'-biphenyl]

| Conditions | Yield |
|---|---|
| With thionyl chloride In N,N-dimethyl-formamide; toluene at 20℃; for 1h; | 98% |
| With thionyl chloride In N,N-dimethyl-formamide; toluene at 20℃; for 1h; | 98% |
| With thionyl chloride In acetonitrile | 87% |
| With thionyl chloride at 20℃; | 77% |
-
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

| Conditions | Yield |
|---|---|
| With phosphorus tribromide In tetrahydrofuran at 20℃; for 0.5h; | 96% |
| With carbon tetrabromide; triphenylphosphine In tetrahydrofuran at 20℃; for 4h; Cooling with ice; | 96% |
| With N-Bromosuccinimide; triphenylphosphine In dichloromethane for 1h; Cooling with ice; | 95% |
-
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

-
-
1061650-37-0
(2-methyl-[1,1′-biphenyl]-3-yl)methanamine

| Conditions | Yield |
|---|---|
| Stage #1: (2-methyl-[1,1'-biphenyl]-3-yl)methanol With hydrazine hydrate In ethanol for 3h; Reflux; Stage #2: With hydrogenchloride In ethanol; water for 0.5h; Reflux; | 96% |
-
-
999-97-3
1,1,1,3,3,3-hexamethyl-disilazane

-
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

-
-
1316808-61-3
trimethyl[(2-methylbiphenyl-3-yl)methoxy]silane

| Conditions | Yield |
|---|---|
| With asymmetric salen type di-Schiff base-based zinc complex supported on Fe3O4 nanoparticles at 20℃; for 0.25h; | 95% |
| With cross-linked poly((30percent)4-vinylpyridine/(70percent)styrene) copolymer-supported bismuth(III) triflate In dichloromethane at 20℃; for 0.166667h; | 91% |
-
-
136918-14-4
phthalimide

-
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

| Conditions | Yield |
|---|---|
| With triphenylphosphine; diethylazodicarboxylate In tetrahydrofuran at 20℃; for 24h; Inert atmosphere; | 92% |
-
-
4489-12-7, 4489-14-9, 26770-95-6, 28684-92-6, 34909-52-9, 51348-74-4, 53955-46-7, 56650-12-5, 14297-81-5
3-(2-methyl-1-propenyl)-2,2-dimethylcyclopropanecarbonyl chloride

-
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

-
-
107686-57-7
(2-methyl-3-phenylphenyl)methyl 2,2-dimethyl-3-(2-methyl-1-propenyl)cyclopropanecarboxylate

| Conditions | Yield |
|---|---|
| In pyridine; diethyl ether for 3h; Ambient temperature; | 85% |
-
-
624-28-2
2,5-dibromopyridine

-
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

| Conditions | Yield |
|---|---|
| With 1,4,7,10,13,20-Hexaoxa<13.1>(1,2)benzenophan; potassium hydroxide In toluene for 1.5h; Reflux; Dean-Stark; | 84% |
-
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

-
-
13223-25-1
2-chloro-4,6-dimethoxypyrimidine

| Conditions | Yield |
|---|---|
| Stage #1: (2-methyl[1,1'-biphenyl]-3-yl)methanol With sodium hydride In N,N-dimethyl-formamide at 0 - 20℃; for 0.5h; Stage #2: 2-chloro-4,6-dimethoxypyrimidine In N,N-dimethyl-formamide at 0 - 20℃; for 2h; | 83% |
-
-
67-56-1
methanol

-
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

| Conditions | Yield |
|---|---|
| With oxygen at 70℃; under 750.075 Torr; for 48h; | 81% |
-
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

-
-
89951-60-0
2-methyl-[1,1'-biphenyl]-3-carbaldehyde

| Conditions | Yield |
|---|---|
| With Dess-Martin periodane In dichloromethane at 20℃; for 0.5h; | 80% |
| With Dess-Martin periodane In dichloromethane for 0.5h; | 57.2% |
| With Dess-Martin periodane In dichloromethane at 20℃; for 0.5h; |
-
-
2973-78-6
3-bromo-4-hydroxybenzylaldehyde

-
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 0℃; for 17h; Inert atmosphere; | 77% |
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 20℃; for 20h; Mitsunobu Displacement; Cooling with ice; | 35% |
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 20℃; for 20h; Cooling with ice; | 35% |
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 0 - 20℃; | 106.9 mg |
-
-
95652-81-6
6-chloro-2-methoxy-3-pyridinecarboxaldehyde

-
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

| Conditions | Yield |
|---|---|
| With palladium diacetate; caesium carbonate; tert-butyl XPhos In toluene at 80℃; Buchwald-Hartwig Coupling; | 71% |
| With palladium diacetate; caesium carbonate; tert-butyl XPhos In toluene at 80℃; for 24h; Inert atmosphere; | 41% |
| With palladium diacetate; caesium carbonate; tert-butyl XPhos In toluene at 80℃; for 4h; Buchwald-Hartwig Coupling; Inert atmosphere; Sealed tube; | 38% |
| With palladium diacetate; caesium carbonate; tert-butyl XPhos In toluene at 80℃; Inert atmosphere; |
-
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

-
-
115363-11-6
2-methyl-[1,1’-biphenyl]-3-carboxylic acid

| Conditions | Yield |
|---|---|
| With sodium bromate; sodium hydrogen sulfate In acetonitrile for 1.5h; Reflux; | 70% |
| With sodium bromate; sodium hydrogensulfate monohydrate In acetonitrile for 1.5h; Reflux; | |
| With Jones reagent In acetone at 25℃; for 6h; | 28 g |

-
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 0℃; Inert atmosphere; | 66% |
-
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

-
-
95-01-2
2,4-Dihydroxybenzaldehyde

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 20℃; Cooling with ice; | 64% |

-
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

| Conditions | Yield |
|---|---|
| With tributylphosphine; 1,1'-azodicarbonyl-dipiperidine In tetrahydrofuran; toluene at 0 - 20℃; for 11h; Mitsunobu Displacement; Inert atmosphere; | 64% |

-
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide; acetonitrile for 11h; Reflux; | 57% |
-
-
22080-96-2
syringaldehyde

-
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 20℃; for 20h; Mitsunobu Displacement; Cooling with ice; | 56% |
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 20℃; for 20h; Cooling with ice; | 56% |
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 23℃; for 18.1667h; Inert atmosphere; | 27% |
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 0 - 20℃; | |
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 0 - 20℃; for 22h; Mitsunobu Displacement; Inert atmosphere; | 699 mg |

-
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 12h; | 53% |

-
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

| Conditions | Yield |
|---|---|
| Stage #1: (2-methyl[1,1'-biphenyl]-3-yl)methanol With sodium hydride In tetrahydrofuran; mineral oil at 0℃; for 0.5h; Stage #2: 5-bromo-4-(4-((tert-butyldimethylsilyl)oxy)butoxy)-2-chloropyrimidine In tetrahydrofuran; mineral oil at 20℃; for 3h; | 49% |

-
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

| Conditions | Yield |
|---|---|
| Stage #1: (2-methyl[1,1'-biphenyl]-3-yl)methanol With sodium hydride In tetrahydrofuran; mineral oil at 0℃; for 0.5h; Stage #2: 5-(((5-bromo-2-chloropyrimidin-4-yl)oxy)methyl)nicotinonitrile In tetrahydrofuran; mineral oil at 20℃; for 3h; | 47% |
-
-
145742-50-3
4-amino-5-chloro-2-methoxybenzaldehyde

-
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile for 18h; Reflux; | 47% |

-
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 12h; | 40.8% |
-
-
78119-82-1
6-hydroxynaphthalene-2-carbaldehyde

-
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 0 - 25℃; | 40% |
| Stage #1: 6-hydroxynaphthalene-2-carbaldehyde; (2-methyl[1,1'-biphenyl]-3-yl)methanol With triphenylphosphine In tetrahydrofuran for 0.25h; Stage #2: With di-isopropyl azodicarboxylate In tetrahydrofuran for 10h; | 3 g |

-
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

| Conditions | Yield |
|---|---|
| Stage #1: (2-methyl[1,1'-biphenyl]-3-yl)methanol With sodium hydride In N,N-dimethyl-formamide at 0℃; for 0.5h; Stage #2: 4-bromo-3-methoxy-1-(methylsulfonyl)-6,7-dihydro-5H-cyclopenta[c]pyridine In N,N-dimethyl-formamide at 20℃; for 6h; | 39% |
-
-
39828-37-0
2,4-dihydroxy-5-methylbenzaldehyde

-
-
76350-90-8
(2-methyl-[1,1'-biphenyl]-3-yl)methanol

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 0 - 40℃; Mitsunobu Displacement; | 37% |
2-Methyl-3-biphenylmethanol Specification
The 2-Methyl-3-biphenylmethanol, with the CAS registry number 76350-90-8, is also known as 3-Hydroxymethyl-2-methylbiphenyl. It belongs to the product categories of Aromatic alcohols and diols; Alkohols; Pesticide; C9 to C30; Oxygen Compounds; Aromatics; Intermediates & Fine Chemicals; Pharmaceuticals. This chemical's molecular formula is C14H14O and molecular weight is 198.26. What's more, its systematic name is (2-Methyl-3-biphenylyl)methanol. This chemical should be sealed and stored in a cool and dry place. Moreover, it should be protected from oxides. It is used as as Bifenthrin intermediate.
Physical properties of 2-Methyl-3-biphenylmethanol are: (1)ACD/LogP: 3.252; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 3.25; (4)ACD/LogD (pH 7.4): 3.25; (5)ACD/BCF (pH 5.5): 174.41; (6)ACD/BCF (pH 7.4): 174.41; (7)ACD/KOC (pH 5.5): 1399.98; (8)ACD/KOC (pH 7.4): 1399.98; (9)#H bond acceptors: 1; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 3; (12)Polar Surface Area: 20.23 Å2; (13)Index of Refraction: 1.587; (14)Molar Refractivity: 62.121 cm3; (15)Molar Volume: 184.81 cm3; (16)Polarizability: 24.627×10-24cm3; (17)Surface Tension: 42.5 dyne/cm; (18)Density: 1.073 g/cm3; (19)Flash Point: 143.013 °C; (20)Enthalpy of Vaporization: 60.541 kJ/mol; (21)Boiling Point: 330.922 °C at 760 mmHg; (22)Vapour Pressure: 0 mmHg at 25°C.
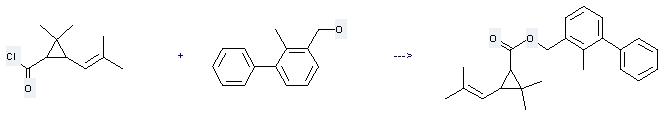
Uses of 2-Methyl-3-biphenylmethanol: it can be used to produce 2,2-dimethyl-3-(2-methyl-propenyl)-cyclopropanecarboxylic acid 2-methyl-biphenyl-3-ylmethyl ester at the ambient temperature. It will need solvents diethyl ether, pyridine with the reaction time of 3 hours. The yield is about 85%.
You can still convert the following datas into molecular structure:
(1)SMILES: OCc2cccc(c1ccccc1)c2C
(2)Std. InChI: InChI=1S/C14H14O/c1-11-13(10-15)8-5-9-14(11)12-6-3-2-4-7-12/h2-9,15H,10H2,1H3
(3)Std. InChIKey: BGTLHJPGBIVQLJ-UHFFFAOYSA-N
Related Products
- 2-Methyl-3-biphenylmethanol
- 7635-11-2
- 76352-01-7
- 7635-28-1
- 7635-29-2
- 7635-45-2
- 7635-46-3
- 7635-54-3
- 76-35-7
- 76359-32-5
- 76360-59-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View