-
Name
2-Naphthalenesulfonyl chloride
- EINECS 202-219-9
- CAS No. 93-11-8
- Article Data51
- CAS DataBase
- Density 1.414 g/cm3
- Solubility insoluble in water
- Melting Point 74-76 °C(lit.)
- Formula C10H7ClO2S
- Boiling Point 371.3 °C at 760 mmHg
- Molecular Weight 226.683
- Flash Point 178.4 °C
- Transport Information UN 3261 8/PG 2
- Appearance light yellow to beige powder
- Safety 26-36/37/39-45-27
- Risk Codes 34
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms 2-Naphthalenylsulfonylchloride;2-Naphthylsulfonyl chloride;NSC 133893;Naphthalene-2-sulfonic acidchloride;b-Naphthalenesulfochloride;2-Naphthalenesulfonylchloride;
- PSA 42.52000
- LogP 3.84810
Synthetic route

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; zirconium(IV) chloride In water; acetonitrile at 25℃; for 0.0166667h; | 98% |
| With N,N,N',N'-tetrachlorobenzene-1,3-disulphonamide; tetrabutyl-ammonium chloride; water In acetonitrile at 0 - 20℃; for 0.333333h; | 98% |
| With trichloroisocyanuric acid; tetrabutylammomium bromide; water In acetonitrile at 0 - 20℃; for 0.333333h; | 98% |

| Conditions | Yield |
|---|---|
| With N,N,N',N'-tetrachlorobenzene-1,3-disulphonamide; tetrabutyl-ammonium chloride; water In acetonitrile at 0 - 20℃; for 0.333333h; | 98% |
| With trichloroisocyanuric acid; water In acetonitrile at 0 - 20℃; for 0.333333h; | 98% |
| With dihydrogen peroxide; zirconium(IV) chloride In water; acetonitrile at 25℃; for 0.0166667h; | 97% |

| Conditions | Yield |
|---|---|
| With trichlorophosphate In chloroform at 90℃; for 9h; Temperature; Solvent; Concentration; | 95.8% |
| With trichlorophosphate; N,N-dimethyl acetamide In sulfolane; acetonitrile at 68 - 72℃; for 0.25h; | 91% |
| With 1,3,5-trichloro-2,4,6-triazine; 18-crown-6 ether In acetone for 20h; Heating; | 89% |
| With chlorine-triphenylphosphine In acetonitrile for 15h; Ambient temperature; |

| Conditions | Yield |
|---|---|
| With 2,2,4,4,6,6-hexachloro-1,3,5-triaza-2,4,6-triphosphorine; potassium chloride at 25℃; for 0.0333333h; Neat (no solvent); | 94% |
| With 1,3,5-trichloro-2,4,6-triazine; triethylamine In acetone for 20h; Heating; | 88% |
| With thionyl chloride In diethyl ether Ambient temperature; | |
| With triethylamine In acetone at 80℃; for 0.333333h; microwave irradiation; |

| Conditions | Yield |
|---|---|
| With 1,3-dichloro-5,5-dimethylhydantoin; acetic acid In dichloromethane; water at 0 - 5℃; Inert atmosphere; | 86% |

| Conditions | Yield |
|---|---|
| With chlorosulfonic acid at 60℃; | 70% |
| With chlorosulfonic acid at 5 - 65℃; |

| Conditions | Yield |
|---|---|
| With N-chloro-succinimide In water; acetonitrile at 35℃; for 0.25h; | A 64% B 27% |

-
-
26189-33-3
2,2-dinaphthylsulfone

-
A
-
91-58-7
2-chloronaphthalene

-
B
-
93-11-8
2-Naphthalenesulfonyl chloride

| Conditions | Yield |
|---|---|
| With phosphorus pentachloride at 180 - 200℃; |

| Conditions | Yield |
|---|---|
| Yield given. Multistep reaction; |

-
-
93-11-8
2-Naphthalenesulfonyl chloride

| Conditions | Yield |
|---|---|
| With phosphorus pentachloride |

-
-
93-11-8
2-Naphthalenesulfonyl chloride

| Conditions | Yield |
|---|---|
| With sulfur dioxide; copper(l) chloride |

-
-
93-11-8
2-Naphthalenesulfonyl chloride

| Conditions | Yield |
|---|---|
| With thionyl chloride; N,N-dimethyl-formamide |

| Conditions | Yield |
|---|---|
| at 200℃; | |
| at 200℃; |
-
-
7719-09-7
thionyl chloride

-
-
120-18-3
naphthalene-2-sulfonate

-
A
-
103858-24-8
naphthalene-2-sulfonic acid-anhydride

-
B
-
93-11-8
2-Naphthalenesulfonyl chloride

-
-
10026-13-8, 874483-75-7
phosphorus pentachloride

-
-
26189-33-3
2,2-dinaphthylsulfone

-
A
-
91-58-7
2-chloronaphthalene

-
B
-
93-11-8
2-Naphthalenesulfonyl chloride

| Conditions | Yield |
|---|---|
| at 180℃; |

-
-
32316-92-0
naphthalene-2-boronic acid

-
-
93-11-8
2-Naphthalenesulfonyl chloride

| Conditions | Yield |
|---|---|
| With phenylchlorosulfate; C43H43NO3PPdS; sodium carbonate In acetone at 50℃; for 12h; Inert atmosphere; Sealed tube; |

| Conditions | Yield |
|---|---|
| Substitution; | 100% |
-
-
582-33-2
3-aminobenzoic acid ethyl ester

-
-
93-11-8
2-Naphthalenesulfonyl chloride

-
-
209173-75-1
ethyl N-(2-naphthalenesulfonyl)-3-aminobenzoate

| Conditions | Yield |
|---|---|
| With dmap In pyridine at 75℃; for 18h; | 100% |
| With triethylamine In tetrahydrofuran |
-
-
885672-69-5
1-(4-chlorophenyl)-N3,N3-dimethylpropane-1,3-diamine

-
-
93-11-8
2-Naphthalenesulfonyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 20℃; for 18h; | 100% |

-
-
93-11-8
2-Naphthalenesulfonyl chloride

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran; mineral oil at 0 - 25℃; for 1h; Inert atmosphere; | 100% |
-
-
124-40-3
dimethyl amine

-
-
93-11-8
2-Naphthalenesulfonyl chloride

-
-
63296-70-8
naphthalene-2-sulfonic acid dimethylamide

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; water at 20℃; for 1.5h; Inert atmosphere; | 99% |
| In tetrahydrofuran; water at 20℃; | 96% |
-
-
93-11-8
2-Naphthalenesulfonyl chloride

-
-
10151-46-9
2-naphthylsulfonyl hydrazide

| Conditions | Yield |
|---|---|
| With hydrazine hydrate In dichloromethane at 0 - 20℃; for 3h; | 99% |
| With hydrazine hydrate In tetrahydrofuran at 0 - 20℃; | 94% |
| With hydrazine hydrate In tetrahydrofuran; water at 0 - 20℃; | 93% |
-
-
944159-28-8
(Z)-2-(5-fluoro-2-methyl-1-(4-(methylthio)benzylidene)-1H-inden-3-yl)-N-methyl-N-(2-(methylamino)ethyl)acetamide

-
-
93-11-8
2-Naphthalenesulfonyl chloride

| Conditions | Yield |
|---|---|
| With pyridine; 1-methyl-1H-imidazole at 0℃; | 99% |
-
-
3850-30-4, 22526-47-2, 59367-75-8, 66228-31-7
(S)-1,2,2-trimethylpropylamine

-
-
93-11-8
2-Naphthalenesulfonyl chloride

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 20℃; for 1.08333h; | 99% |
-
-
462-06-6
fluorobenzene

-
-
93-11-8
2-Naphthalenesulfonyl chloride

-
-
117953-29-4
βa-naphthyl p-fluorophenyl sulphone

| Conditions | Yield |
|---|---|
| With iron(III) chloride at 20℃; Temperature; | 98.9% |
-
-
62-53-3
aniline

-
-
93-11-8
2-Naphthalenesulfonyl chloride

-
-
1576-48-3
naphthalene-2-sulfonic acid phenylamide

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 20℃; for 4h; | 98% |
| With triethylamine In dichloromethane at 20℃; Substitution; | 95% |
| With triethylamine In dichloromethane at 0 - 20℃; for 24h; Inert atmosphere; | 83% |

| Conditions | Yield |
|---|---|
| With tungsten(VI) chloride; sodium iodide In acetonitrile for 16h; Ambient temperature; | 98% |
| With Silphos; iodine In acetonitrile for 8h; Heating; | 98% |
| With aluminium(III) iodide In acetonitrile 1.) room temp., 4 h; 2.) reflux, 1 h; | 95% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; tetra(n-butyl)ammonium hydrogensulfate; potassium carbonate In benzene Substitution; | 98% |

| Conditions | Yield |
|---|---|
| In 1,4-dioxane at 20℃; | 98% |
-
-
109-89-7
diethylamine

-
-
93-11-8
2-Naphthalenesulfonyl chloride

-
-
6307-08-0
N,N-diethylnaphthalene-2-sulfonamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 1h; | 97% |
| With potassium hydroxide |

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 20℃; for 3h; Substitution; | 97% |

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine; hydrogenchloride In tetrahydrofuran; dichloromethane | 97% |
| With 4-methyl-morpholine; hydrogenchloride In tetrahydrofuran; dichloromethane | 97% |
| With 4-methyl-morpholine; hydrogenchloride In tetrahydrofuran; dichloromethane | 97% |
| With pyridine In dichloromethane at 20℃; |

-
-
93-11-8
2-Naphthalenesulfonyl chloride

| Conditions | Yield |
|---|---|
| Stage #1: (+)-(2R,6S)-3-[(1S,2R)-methoxy-7,7-dimethylbicyclo[2.2.1]heptane-1-carbonyl]-3,5-diazadibenzo[h,k]tricyclo[5.2.2.22,6]undeca-8,10-dien-4-one With sodium hydride In tetrahydrofuran; mineral oil at 0℃; for 0.166667h; Stage #2: 2-Naphthalenesulfonyl chloride In tetrahydrofuran; mineral oil at 0 - 20℃; | 97% |

-
-
93-11-8
2-Naphthalenesulfonyl chloride

| Conditions | Yield |
|---|---|
| With pyridine; 1-methyl-1H-imidazole at 0℃; | 97% |
-
-
53981-25-2
2-amino-6-fluoro phenol

-
-
93-11-8
2-Naphthalenesulfonyl chloride

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 20℃; for 4h; | 97% |
-
-
197069-37-7
(1R,2R,3S,5S)-(5Z)-7-(2-amino-6,6-dimethylbicyclo[3.1.1]hept-3-yl)-hept-5-enoic acid methyl ester

-
-
93-11-8
2-Naphthalenesulfonyl chloride

-
-
120632-51-1
methyl (5Z)-7-((1R,2R,3S,5S)-2-(2-naphthyl sulfonylamino)-6,6-dimethylbicyclo<3.1.1>hept-3-yl)hept-5-enoate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane for 1h; Ambient temperature; | 96.3% |
-
-
93-11-8
2-Naphthalenesulfonyl chloride

-
-
63735-42-2
sodium naphthalen-2-ylsulfinate

| Conditions | Yield |
|---|---|
| With hydrazine hydrate; sodium carbonate In water at 80℃; for 2h; | 96.3% |
| With sodium hydrogencarbonate; sodium sulfite In water at 80℃; for 4h; | 91% |
| With sodium dithionite; sodium carbonate In water at 40℃; for 2h; | 54% |
-
-
93-11-8
2-Naphthalenesulfonyl chloride

-
-
74-89-5
methylamine

-
-
7147-68-4
N-methyl-2-naphthalenesulfonamide

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 20℃; for 16h; Inert atmosphere; | 96% |
| With diethyl ether | |
| With pyridine In dichloromethane at 0 - 20℃; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With hydrogen; carbon monoxide; Sn; silica gel In chlorobenzene at 96.9℃; under 12751 Torr; for 4h; | 96% |
| With water; zinc at 60 - 70℃; Behandeln des Reaktionsprodukts mit verd. Salzsaeure und dann mit Zinkstaub; | |
| With lithium aluminium tetrahydride; diethyl ether |
-
-
93-11-8
2-Naphthalenesulfonyl chloride

-
-
714567-80-3
2-[(2R)-3-Oxo-1,2,3,4-tetrahydroquinoxalin-2-yl]-N-[2-(4-cyanophenyl)ethyl]acetamide

| Conditions | Yield |
|---|---|
| With pyridine | 96% |
-
-
68076-38-0
N1-benzyloxycarbonyl-N3-tert-butyloxycarbonyl-spermidine

-
-
93-11-8
2-Naphthalenesulfonyl chloride

-
-
461441-09-8
N1-benzyloxycarbonyl-N3-tert-butyloxycarbonyl-N2-2-naphthalenesulfonyl-spermidine

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane for 20h; | 96% |
-
-
93-11-8
2-Naphthalenesulfonyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 42.75h; | 96% |
-
-
5856-63-3
(2R)-2-aminobutan-1-ol

-
-
93-11-8
2-Naphthalenesulfonyl chloride

-
-
1204571-32-3
(R)-2-(2-naphthalensulfonylamino)-2-ethyl-1-ethanol

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 15.5h; | 96% |
-
-
41979-39-9
4-piperidone hydrochloride

-
-
93-11-8
2-Naphthalenesulfonyl chloride

-
-
1225198-43-5
1-(naphthalen-2-ylsulfonyl)-4-piperidinone

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 0 - 20℃; | 96% |
2-Naphthalenesulfonyl Chloride Specification
The 2-Naphthalenesulfonyl Chloride is an organic compound with the formula C10H7ClO2S. The IUPAC name of this chemical is naphthalene-2-sulfonyl chloride. With the CAS registry number 93-11-8 and EINECS 202-219-9, it is also named as Naphthalene-2-sulfonic acid chloride. The product's categories are Organic Building Blocks; Sulfonyl Halides; Sulfur Compounds. It is light yellow to beige powder which is insoluble in water and sensitive to moisture. In addition, this chemical should be sealed in the container and stored in the dry place.
The other characteristics of this product can be summarized as: (1)ACD/LogP: 3.22; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 3.22; (4)ACD/LogD (pH 7.4): 3.22; (5)ACD/BCF (pH 5.5): 164.3; (6)ACD/BCF (pH 7.4): 164.3; (7)ACD/KOC (pH 5.5): 1341.39; (8)ACD/KOC (pH 7.4): 1341.39; (9)#H bond acceptors: 2; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 42.52 Å2; (13)Index of Refraction: 1.643; (14)Molar Refractivity: 57.95 cm3; (15)Molar Volume: 160.2 cm3; (16)Polarizability: 22.97×10-24 cm3; (17)Surface Tension: 50.5 dyne/cm; (18)Density: 1.414 g/cm3; (19)Flash Point: 178.4 °C; (20)Enthalpy of Vaporization: 59.4 kJ/mol; (21)Boiling Point: 371.3 °C at 760 mmHg; (22)Vapour Pressure: 2.23E-05 mmHg at 25°C.
Preparation of 2-Naphthalenesulfonyl Chloride: It can be obtained by naphthalene-2-sulfonic acid ; sodium-salt. This reaction needs reagent POCl3, catalytic agent N,N-Dimethylacetamide and solvents acetonitrile, tetrahydrothiophene 1,1-dioxide at temperature of 68-72 °C. The reaction time is 15 min. The yield is 91%.
.gif)
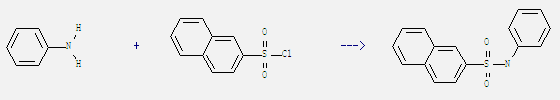
Uses of 2-Naphthalenesulfonyl Chloride: It is used in the determination of amines and used in organic synthesis. For example: it can react with aniline to get naphthalene-2-sulfonanilide. This reaction needs reagent Et3N and solvent CH2Cl2 at temperature of 20 °C. The yield is 95%.
When you are using this chemical, please be cautious about it as the following:
It can cause burns. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. If you want to contact this product, you must wear suitable protective clothing, gloves and eye/face protection. In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
People can use the following data to convert to the molecule structure.
1. SMILES:ClS(=O)(=O)c2ccc1c(cccc1)c2
2. InChI:InChI=1/C10H7ClO2S/c11-14(12,13)10-6-5-8-3-1-2-4-9(8)7-10/h1-7H
3. InChIKey:OPECTNGATDYLSS-UHFFFAOYAP
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View