-
Name
ALPHA-METHYLPHENYLACETONITRILE
- EINECS 217-354-9
- CAS No. 1823-91-2
- Article Data228
- CAS DataBase
- Density 0.987 g/cm3
- Solubility
- Melting Point 210 °C(Solv: ethyl ether (60-29-7); chloroform (67-66-3))
- Formula C9H9N
- Boiling Point 231 °C at 760 mmHg
- Molecular Weight 131.177
- Flash Point 93.9 °C
- Transport Information UN 3276 6.1/PG 3
- Appearance clear very slightly yellow liquid
- Safety 26-27-37/39
- Risk Codes 20/21/22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn,  Xi
Xi
- Synonyms Hydratroponitrile(6CI,7CI,8CI);NSC 11264;NSC 37485;dl-2-Phenylpropionitrile;dl-a-Phenylethyl isocyanide;a-Cyanoethylbenzene;a-Methylbenzeneacetonitrile;a-Methylbenzyl cyanide;a-Methylphenylacetonitrile;a-Phenylethyl cyanide;a-Phenylpropionitrile;
- PSA 23.79000
- LogP 2.31368
Synthetic route

| Conditions | Yield |
|---|---|
| Stage #1: propiononitrile With n-butyllithium In tetrahydrofuran at -78℃; for 0.25h; Stage #2: Phenyl[2-(trimethylsilyl)phenyl]iodonium trifluoromethanesulfonate With tetrabutyl ammonium fluoride In tetrahydrofuran at 0 - 20℃; for 0.916667h; | 99% |
-
-
59647-78-8
2-phenylpropanal oxime

-
-
1823-91-2
1-phenylethyl cyanide

| Conditions | Yield |
|---|---|
| With copper diacetate In acetonitrile for 3h; Reflux; | 98% |
| With pyridine; titanium tetrachloride at 40℃; Sealed tube; | 93% |
| With acetyl chloride; zinc(II) oxide for 0.5h; Heating; | 90% |
-
-
34713-70-7
2-Phenylpropanal

-
-
1823-91-2
1-phenylethyl cyanide

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid; O-benzenesulfonyl-acetohydroxamic acid ethyl ester In dichloromethane at 23℃; for 24h; Inert atmosphere; | 98% |
| With pyridine; hydroxylamine hydrochloride; titanium tetrachloride at 40℃; Sealed tube; | 97% |
| With aluminum oxide; hydroxylamine hydrochloride; methanesulfonyl chloride at 100℃; for 0.5h; | 95% |

-
-
1823-91-2
1-phenylethyl cyanide

| Conditions | Yield |
|---|---|
| With iron(III) chloride; 2,6-di-tert-butyl-4-methyl-phenol In toluene at 20℃; for 0.0833333h; Schlenk technique; | 97% |
-
-
10442-39-4
tetra-n-butylammonium cyanide

-
-
105966-39-0
2-(1-phenylethoxy)-tetrahydro-2H-pyran

-
-
1823-91-2
1-phenylethyl cyanide

| Conditions | Yield |
|---|---|
| With triphenylphosphine; 2,3-dicyano-5,6-dichloro-p-benzoquinone In acetonitrile for 6h; Heating; | 94% |
-
-
69573-35-9
2-chloro-2-phenylpropanenitrile

-
-
1823-91-2
1-phenylethyl cyanide

| Conditions | Yield |
|---|---|
| With sodium hydrogen telluride; acetic acid In ethanol; benzene at -20℃; for 0.333333h; | 93% |

| Conditions | Yield |
|---|---|
| With aluminium trichloride; potassium iodide In water; acetonitrile at 80℃; for 10h; | 92% |
| With phosgene In tetrahydrofuran; toluene at 25℃; for 4h; Inert atmosphere; | 82% |
| With phosphorus pentoxide; benzene | |
| With phosphorus pentachloride |
-
-
140-29-4
phenylacetonitrile

-
-
74-88-4
methyl iodide

-
A
-
1195-98-8
2-methyl-2-phenylpropionitrile

-
B
-
1823-91-2
1-phenylethyl cyanide

| Conditions | Yield |
|---|---|
| With sodium hydroxide; tetrakis(hexylsulfinylmethyl)methane for 28h; Ambient temperature; | A 5% B 92% |
| With sodium hydroxide; C(CH2S(=O)C6H13)4 for 28h; Ambient temperature; | A 5% B 92% |
| With sodium hydroxide; sulfur dioxide for 24h; Product distribution; Ambient temperature; liquid-liquid two phase alkylation, other alkyl iodides, other sulfoxides containing pyridine nuklei as cat.; | A n/a B 83 % Chromat. |

-
-
127462-20-8, 25438-38-4
2-phenyl-2-trimethylsilyloxypropanenitrile

-
-
1823-91-2
1-phenylethyl cyanide

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane; water; sodium iodide In acetonitrile at 20℃; for 48h; Reduction; | 92% |
| With chloro-trimethyl-silane; water; acetonitrile; sodium iodide In hexane for 24h; Ambient temperature; | 74% |

| Conditions | Yield |
|---|---|
| Stage #1: potassium cyanide With acetic acid In ethylene glycol at 60℃; Sealed tube; Stage #2: styrene With bis(1,5-cyclooctadiene)nickel (0); 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene at 60℃; for 18h; Sealed tube; | 92% |
-
-
98-85-1, 13323-81-4
1-Phenylethanol

-
-
10442-39-4
tetra-n-butylammonium cyanide

-
-
1823-91-2
1-phenylethyl cyanide

| Conditions | Yield |
|---|---|
| With triphenylphosphine; 2,3-dicyano-5,6-dichloro-p-benzoquinone In acetonitrile at 20℃; | 91% |

| Conditions | Yield |
|---|---|
| With potassium carbonate at 180℃; for 3.75h; | 90% |
| With sodium methylate at 180℃; under 15001.5 Torr; for 5h; Reagent/catalyst; Temperature; | 88.2% |
| aluminum oxide; Corundum(Al2O3) + 5wtpercent K2CO3 + 5wtpercent polyethyleneglycol (mol. weight 6000); potassium carbonate at 180℃; liquid flow rate 16 ml/h; | 88 % Chromat. |
-
-
7677-24-9
trimethylsilyl cyanide

-
-
98-85-1, 13323-81-4
1-Phenylethanol

-
-
1823-91-2
1-phenylethyl cyanide

| Conditions | Yield |
|---|---|
| With tris(pentafluorophenyl)borate In acetonitrile at 20℃; for 1h; | 90% |
| With zinc trifluoromethanesulfonate In nitromethane at 100℃; for 8h; | 43% |

| Conditions | Yield |
|---|---|
| With fluorescein sodium salt In acetonitrile at 20℃; for 10h; Irradiation; | 90% |

| Conditions | Yield |
|---|---|
| With bis(1,5-cyclooctadiene)nickel (0); 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In toluene at 120℃; for 24h; Inert atmosphere; Glovebox; Sealed tube; regioselective reaction; | 90% |

| Conditions | Yield |
|---|---|
| With 3-[bis-(4-methoxy-phenyl)-phosphanyl]-2H-isoquinolin-1-one; N-{6-[bis-(4-methoxy-phenyl)-phosphanyl]-pyridin-2-yl}-2,2-dimethyl-propionamide; bis(1,5-cyclooctadiene)nickel (0) In toluene at 35℃; for 25h; regioselective reaction; | 89% |
| With bis(1,5-cyclooctadiene)nickel (0); (S)-binaphthyl chiral diphosphite In toluene at 20℃; for 24h; asymmetric hydrocyanation; |

| Conditions | Yield |
|---|---|
| With (1,2-dimethoxyethane)dichloronickel(II); (1S,2R)-(+)-norphedrine; lithium hexamethyldisilazane; water; cesium fluoride In N,N-dimethyl acetamide at 60℃; for 16h; Hiyama cross-coupling; | 88% |
-
-
31600-55-2
benzyl methoxymethyl ether

-
-
94073-86-6
(1-methoxymethoxy)ethylbenzene

-
-
10442-39-4
tetra-n-butylammonium cyanide

-
A
-
140-29-4
phenylacetonitrile

-
B
-
1823-91-2
1-phenylethyl cyanide

| Conditions | Yield |
|---|---|
| With 1-(n-butyl)-3-methylimidazolium tetrachloroindate at 135 - 140℃; for 0.075h; Microwave irradiation; Neat (no solvent); chemoselective reaction; | A 88% B 19% |

-
-
492-37-5, 2328-24-7
hydratropic acid

-
-
1823-91-2
1-phenylethyl cyanide

| Conditions | Yield |
|---|---|
| With sodium azide; triethylamine; triphenylphosphine; (bis-(2-methoxyethyl)amino)sulfur trufluoride In dichloromethane; dimethyl sulfoxide at 0 - 20℃; for 0.833333h; | 87% |
| With sodium azide; TEA; triethylphosphine; (bis-(2-methoxyethyl)amino)sulfur trufluoride In dichloromethane; dimethyl sulfoxide at 0℃; for 30h; | 85% |
| Multi-step reaction with 3 steps 1: thionyl chloride / 0.5 h / 25 °C / Inert atmosphere 2: ammonia / water / 0.5 h / 25 °C 3: phosgene / toluene; tetrahydrofuran / 4 h / 25 °C / Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| With carbon monoxide; hydrogen; dodecacarbonyl-triangulo-triruthenium In N,N-dimethyl-formamide at 230℃; under 76000.1 Torr; for 16h; other active methylene compounds, other aldehydes; | 86% |
| With carbon monoxide; hydrogen; dodecacarbonyl-triangulo-triruthenium In N,N-dimethyl-formamide at 230℃; under 76000.1 Torr; for 16h; | 86% |

| Conditions | Yield |
|---|---|
| With bis(η3-allyl-μ-chloropalladium(II)); 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In 1,3,5-trimethyl-benzene at 20 - 140℃; for 10.1667h; Inert atmosphere; Sealed tube; chemoselective reaction; | 86% |

| Conditions | Yield |
|---|---|
| With cobalt(II) tetrafluoroborate hexahydrate; tris(2-diphenylphosphinoethyl)phosphine; potassium carbonate at 100℃; for 24h; Autoclave; Glovebox; Inert atmosphere; | 85% |
| With [carbonylchlorohydrido{bis[2-(diphenylphosphinomethyl)ethyl]amino}ethylamino] ruthenium(II); potassium tert-butylate In toluene at 135℃; for 4h; Inert atmosphere; | 83% |
| With bis(1,5-cyclooctadiene)diiridium(I) dichloride; potassium tert-butylate; 1,2-bis-(diphenylphosphino)ethane at 150℃; for 48h; Inert atmosphere; | 80% |

| Conditions | Yield |
|---|---|
| With sodium cyanide; N-benzyl-N,N,N-triethylammonium chloride In acetonitrile at 35℃; for 45h; | 83% |

| Conditions | Yield |
|---|---|
| With trimethylamine-borane; 1,3,4,6,7,8-hexahydro-2H-pyrimido[1,2-a]pyrimidine; potassium tert-butylate In 1,2-dimethoxyethane at 120℃; for 16h; Sealed tube; | 83% |

| Conditions | Yield |
|---|---|
| With (3S,4S)-1-benzyl-3,4-pyrrolidin-diol-onium iodide; potassium carbonate In N,N-dimethyl-formamide for 12h; Ambient temperature; | 82% |

-
-
1823-91-2
1-phenylethyl cyanide

| Conditions | Yield |
|---|---|
| IrH5(P-(i-Pr)3)2 In toluene at 150℃; for 12h; | 82% |
-
-
292638-84-7
styrene

-
-
100-47-0
benzonitrile

-
A
-
588-59-0
stilbene

-
B
-
645-59-0
dihydrocinnamonitrile

-
C
-
1823-91-2
1-phenylethyl cyanide

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); dimethylaluminum chloride; CyJohnPhos In hexane; toluene at 100℃; for 16h; Inert atmosphere; Glovebox; regioselective reaction; | A 82% B 57 %Chromat. C 13 %Chromat. |


| Conditions | Yield |
|---|---|
| With bis(1,5-cyclooctadiene)nickel (0); 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In benzene at 90℃; for 18h; Catalytic behavior; Reagent/catalyst; Solvent; Temperature; Schlenk technique; Inert atmosphere; Glovebox; regioselective reaction; | 82% |

| Conditions | Yield |
|---|---|
| With bis(acetylacetonate)nickel(II); 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene; zinc at 150℃; for 24h; Reagent/catalyst; Sealed tube; | 81% |
| With bis(acetylacetonate)nickel(II); 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene; zinc for 24h; Mechanism; Reagent/catalyst; Inert atmosphere; Sealed tube; Heating; Green chemistry; regioselective reaction; | 78% |
-
-
591-87-7
Allyl acetate

-
-
1823-91-2
1-phenylethyl cyanide

-
-
104367-49-9
2-methyl-2-phenyl-4-pentenenitrile

| Conditions | Yield |
|---|---|
| With potassium hydroxide; Pd(PhCH=CH)2CO>2CO; 1,2-bis-(diphenylphosphino)ethane In dichloromethane Ambient temperature; | 100% |
-
-
66003-76-7
Diphenyliodonium triflate

-
-
1823-91-2
1-phenylethyl cyanide

-
-
19341-03-8
N-phenyl-2-phenylpropanamide

| Conditions | Yield |
|---|---|
| With copper (I) trifluoromethane sulfonate toluene complex; caesium carbonate In toluene at 80℃; for 0.333333h; Catalytic behavior; Mechanism; Solvent; Reagent/catalyst; Temperature; | 100% |
-
-
106-95-6
allyl bromide

-
-
1823-91-2
1-phenylethyl cyanide

-
-
104367-49-9
2-methyl-2-phenyl-4-pentenenitrile

| Conditions | Yield |
|---|---|
| With sodium hexamethyldisilazane In tetrahydrofuran; dimethyl sulfoxide for 0.666667h; | 99.5% |
| Stage #1: 1-phenylethyl cyanide With lithium hexamethyldisilazane In tetrahydrofuran at -78℃; Stage #2: allyl bromide In tetrahydrofuran at -78 - 20℃; | |
| Stage #1: 1-phenylethyl cyanide With n-butyllithium; diisopropylamine In tetrahydrofuran; hexane at 0℃; for 0.5h; Stage #2: allyl bromide In tetrahydrofuran; hexane at 0 - 20℃; |
-
-
1823-91-2
1-phenylethyl cyanide

-
-
492-37-5, 2328-24-7
hydratropic acid

| Conditions | Yield |
|---|---|
| With recombinant nitrilase from Pseudomonas fluorescens EBC 191 In methanol; phosphate buffer at 25℃; pH=6; | 99% |
| With water; sodium hydroxide at 120℃; for 6h; Temperature; | 97% |
| Stage #1: 1-phenylethyl cyanide With sodium hydroxide at 105℃; for 12.5h; Reflux; Stage #2: With sulfuric acid for 0.583333h; pH=3; Temperature; pH-value; | 93% |

| Conditions | Yield |
|---|---|
| With dichloro( 1,5-cyclooctadiene)platinum(ll); 4,5-dihydro-3H-dinaphtho[2,1-c:1',2'-e]phosphepine-4-oxide; water; silver nitrate In tert-Amyl alcohol at 80℃; for 1h; Catalytic behavior; Reagent/catalyst; Time; chemoselective reaction; | 99% |
| With [RuH(tBu-PNP(-))(CO)]; water In tert-butyl alcohol at 20℃; for 24h; | 99% |
| With sodium hydroxide; dihydrogen peroxide In methanol; water for 2h; pH=8.0; | 95% |
-
-
64-17-5
ethanol

-
-
1823-91-2
1-phenylethyl cyanide

-
-
94521-90-1
ethyl 2-methyl-2-phenylacetoimidate hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 0 - 5℃; Addition; | 99% |
| With hydrogenchloride In diethyl ether at 20℃; | 84% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-phenylethyl cyanide With lithium diisopropyl amide In tetrahydrofuran at -78℃; for 0.333333h; Stage #2: 1-azido-3-chlorobenzene With N,N,N,N,N,N-hexamethylphosphoric triamide In tetrahydrofuran at -78 - 20℃; for 1h; Stage #3: dimethyl sulfate at 20℃; for 1h; | 99% |


| Conditions | Yield |
|---|---|
| Stage #1: 1-phenylethyl cyanide With lithium diisopropyl amide In tetrahydrofuran at -78℃; for 0.333333h; Stage #2: 3-methoxyphenyl azide With N,N,N,N,N,N-hexamethylphosphoric triamide In tetrahydrofuran at -78 - 20℃; for 1h; Stage #3: dimethyl sulfate at -78℃; for 1h; | 99% |


| Conditions | Yield |
|---|---|
| With samarium diiodide; water; triethylamine In tetrahydrofuran at 20℃; for 0.0833333h; Inert atmosphere; | 98% |
| With hydrogenchloride; ethanol; palladium Hydrogenation; | |
| With ethanol; ammonia; nickel Hydrogenation; | |
| With lithium aluminium tetrahydride In diethyl ether | |
| Multi-step reaction with 2 steps 1: woollins’ reagent / toluene / 6 h / Inert atmosphere; Reflux 2: water; samarium diiodide / tetrahydrofuran / 20 °C / Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| With ammonia; sodium amide at -70℃; for 0.0333333h; | 98% |
-
-
100-39-0
benzyl bromide

-
-
1823-91-2
1-phenylethyl cyanide

-
-
5558-92-9
α-benzyl-α-methylphenylacetonitrile

| Conditions | Yield |
|---|---|
| Stage #1: 1-phenylethyl cyanide With n-butyllithium; diisopropylamine In tetrahydrofuran; hexane at 0℃; for 0.5h; Stage #2: benzyl bromide In tetrahydrofuran; hexane at 0 - 20℃; | 98% |
| Stage #1: 1-phenylethyl cyanide With lithium diisopropyl amide In tetrahydrofuran at -78℃; for 0.666667h; Stage #2: benzyl bromide In tetrahydrofuran at -78 - 20℃; for 6h; |
-
-
1823-91-2
1-phenylethyl cyanide

-
-
16428-57-2
2-deuterio-2-phenyl-propionitrile

| Conditions | Yield |
|---|---|
| With water-d2; triethylamine at 20℃; for 72h; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-phenylethyl cyanide With lithium diisopropyl amide In tetrahydrofuran at -78℃; for 0.333333h; Stage #2: 3-methoxyphenyl azide With N,N,N,N,N,N-hexamethylphosphoric triamide In tetrahydrofuran at -78 - 20℃; for 1h; Stage #3: diethyl sulfate at 20℃; for 1h; | 97% |

-
-
1823-91-2
1-phenylethyl cyanide

| Conditions | Yield |
|---|---|
| With trimethylaluminum; ammonium chloride In toluene at 20 - 80℃; for 17h; | 95% |
| With trimethylaluminum; ammonium chloride In toluene at 80℃; for 48h; | 92% |
-
-
13154-24-0
triisopropylsilyl chloride

-
-
1823-91-2
1-phenylethyl cyanide

| Conditions | Yield |
|---|---|
| Stage #1: 1-phenylethyl cyanide With n-butyllithium; diisopropylamine In tetrahydrofuran; hexane at -78℃; for 0.0833333h; Inert atmosphere; Stage #2: triisopropylsilyl chloride In tetrahydrofuran; hexane at -78 - 20℃; for 12h; Inert atmosphere; | 95% |
-
-
107-13-1
acrylonitrile

-
-
1823-91-2
1-phenylethyl cyanide

-
-
14149-35-0
(+/-)-3-Methyl-3-phenyl-2,6-piperidinedione

| Conditions | Yield |
|---|---|
| With IrH5(P-(i-Pr)3)2; water In tetrahydrofuran at 150℃; for 20h; | 94% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-phenylethyl cyanide With lithium diisopropyl amide In tetrahydrofuran at -78℃; for 0.333333h; Stage #2: 1-azido-4-chlorobenzene With N,N,N,N,N,N-hexamethylphosphoric triamide In tetrahydrofuran at -78 - 20℃; for 1h; Stage #3: dimethyl sulfate at 20℃; for 1h; | 94% |


| Conditions | Yield |
|---|---|
| Stage #1: 1-phenylethyl cyanide With lithium diisopropyl amide In tetrahydrofuran at -78℃; for 0.333333h; Stage #2: 1-azido-4-bromobenzene With N,N,N,N,N,N-hexamethylphosphoric triamide In tetrahydrofuran at -78 - 20℃; for 1h; Stage #3: dimethyl sulfate at 20℃; for 1h; | 94% |

-
-
3376-23-6, 7372-59-0, 59862-60-1
C-phenyl-N-methylnitrone

-
-
79271-56-0
Triethylsilyl trifluoromethanesulfonate

-
-
1823-91-2
1-phenylethyl cyanide

| Conditions | Yield |
|---|---|
| With triethylamine In 1,2-dichloro-ethane at -30℃; for 0.5h; Inert atmosphere; | 94% |

-
-
1823-91-2
1-phenylethyl cyanide

-
-
513-42-8
3-hydroxy-2-methyl-1-propene

-
-
1199790-77-6
2,4-dimethyl-2-phenyl-4-pentenenitrile

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); carbon dioxide In dimethyl sulfoxide at 90℃; under 760.051 Torr; for 14h; Sealed tube; | 94% |

-
-
1823-91-2
1-phenylethyl cyanide

-
-
1352055-05-0
2-methyl-3-(naphthalen-2-yl)-2-phenylpropanenitrile

| Conditions | Yield |
|---|---|
| With bis(1,5-cyclooctadiene)nickel(0); 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl; lithium tert-butoxide In toluene at 100℃; for 12h; | 94% |
-
-
611-94-9
4-Methoxybenzophenone

-
-
1823-91-2
1-phenylethyl cyanide

-
-
33092-22-7
2-(4-benzoylphenyl)-2-phenylpropanenitrile

| Conditions | Yield |
|---|---|
| With phosphazene base-P4-tert-butyl In tetrahydrofuran; hexane at 60℃; for 18h; Catalytic behavior; Reagent/catalyst; Solvent; Temperature; Inert atmosphere; Molecular sieve; Sealed tube; | 93% |
2-Phenylpropionitrile Specification
The Benzeneacetonitrile, α-methyl-, with the CAS registry number 1823-91-2, is also known as alpha-Methylbenzyl cyanide. Its EINECS registry number is 217-354-9. This chemical's molecular formula is C9H9N and molecular weight is 131.17. Its IUPAC name is called 2-phenylpropanenitrile. The product should be sealed and stored in cool, dry place. What's more, it should be protected from strong oxides.
Physical properties of Benzeneacetonitrile, α-methyl-: (1)ACD/LogP: 1.80; (2)ACD/LogD (pH 5.5): 1.8; (3)ACD/LogD (pH 7.4): 1.8; (4)ACD/BCF (pH 5.5): 13.65; (5)ACD/BCF (pH 7.4): 13.65; (6)ACD/KOC (pH 5.5): 226.06; (7)ACD/KOC (pH 7.4): 226.06; (8)#H bond acceptors: 1; (9)#Freely Rotating Bonds: 1; (10)Index of Refraction: 1.519; (11)Molar Refractivity: 40.35 cm3; (12)Molar Volume: 132.8 cm3; (13)Surface Tension: 38.3 dyne/cm; (14)Density: 0.987 g/cm3; (15)Flash Point: 93.9 °C; (16)Enthalpy of Vaporization: 46.76 kJ/mol; (17)Boiling Point: 231 °C at 760 mmHg; (18)Vapour Pressure: 0.0639 mmHg at 25°C.
Preparation of Benzeneacetonitrile, α-methyl-: this chemical can be prepared by phenylacetonitrile and iodomethane. This reaction will need reagent LDA and solvent diethyl ether. The reaction temperature is -90 °C. The yield is about 55%.

Uses of Benzeneacetonitrile, α-methyl-: it can be used to produce 3-cyano-3-phenyl-butyric acid ethyl ester at temperature of 60 °C. This reaction will need reagent NaNH2 and solvent toluene.
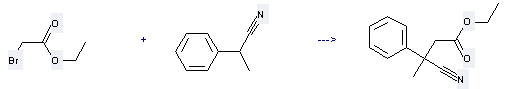
When you are using this chemical, please be cautious about it as the following:
This chemical may cause inflammation to the skin or other mucous membranes. It is irritating to eyes, respiratory system and skin. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. Whenever you will contact it, please wear suitable protective gloves and eye/face protection.
You can still convert the following datas into molecular structure:
(1)Canonical SMILES: CC(C#N)C1=CC=CC=C1
(2)InChI: InChI=1S/C9H9N/c1-8(7-10)9-5-3-2-4-6-9/h2-6,8H,1H3
(3)InChIKey: NVAOLENBKNECGF-UHFFFAOYSA-N
Related Products
- 2-Phenylpropionitrile
- 18240-73-8
- 18240-93-2
- 182410-00-0
- 18241-31-1
- 182415-24-3
- 18241-63-9
- 182417-07-8
- 18242-39-2
- 18242-99-4
- 18243-23-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View