-
Name
2-Benzylaminopyridine
- EINECS 230-060-5
- CAS No. 6935-27-9
- Article Data178
- CAS DataBase
- Density 1.131 g/cm3
- Solubility
- Melting Point 95-97 °C(lit.)
- Formula C12H12N2
- Boiling Point 321.8 °C at 760 mmHg
- Molecular Weight 184.241
- Flash Point 148.4 °C
- Transport Information
- Appearance white to off-white powder
- Safety 26-39
- Risk Codes 22-37/38-41
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Pyridine,2-(benzylamino)- (6CI,7CI,8CI);2-(Benzylamino)pyridine;N-Benzyl-2-aminopyridine;N-Benzyl-2-pyridinamine;N-Benzylpyridine-2-amine;NSC 59833;
- PSA 24.92000
- LogP 2.76670
Synthetic route

| Conditions | Yield |
|---|---|
| With dichloro-[1,3-bis(4-tert-butylbenzyl)perhydrobenzimidazol-2-ylidene](p-cymene)ruthenium(II); potassium tert-butylate at 120℃; for 24h; Reagent/catalyst; Inert atmosphere; Schlenk technique; Sealed tube; | 100% |
| With potassium tert-butylate; copper diacetate In 1,4-dioxane at 130℃; for 24h; | 99% |
| With potassium tert-butylate; copper diacetate In 1,4-dioxane at 130℃; for 24h; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With sodium t-butanolate In toluene at 100 - 110℃; for 24h; Buchwald-Hartwig amination; Inert atmosphere; Sealed vial; | 99% |
| With potassium phosphate In dimethyl sulfoxide at 80℃; UV-irradiation; | 95% |
| With copper(l) iodide; C14H16N2O; caesium carbonate In N,N-dimethyl-formamide at 130℃; for 24h; Inert atmosphere; Sealed tube; | 93% |

| Conditions | Yield |
|---|---|
| With cesium acetate; copper In dimethyl sulfoxide at 90℃; for 24h; Inert atmosphere; | 99% |
| With copper(l) iodide; 1-tetralone oxime; potassium hydroxide In water at 25℃; for 18h; Sealed tube; Inert atmosphere; Green chemistry; | 93% |
| With tetra(n-butyl)ammonium hydroxide; copper(II) sulfate In water at 80℃; for 24h; Schlenk technique; Sealed tube; Inert atmosphere; | 80% |
| With tetramethyl ammoniumhydroxide; ethyl 2-oxocyclohexane carboxylate; copper(l) chloride In dimethyl sulfoxide at 80℃; for 24h; Inert atmosphere; | 72% |
-
-
1883-96-1, 82299-15-8, 88785-73-3
N-benzylidenepyridin-2-amine

-
-
6935-27-9
N-benzylpyridin-2-amine

| Conditions | Yield |
|---|---|
| With Triethoxysilane; [bis(2,6-diisopropylaniline)acenaphthene]Fe(η6-toluene) at 70℃; for 18h; Schlenk technique; Inert atmosphere; Glovebox; Sealed tube; | 98% |
| With (4-Ph)Triaz(NHPiPr2)2Mn(CO)2Br; potassium tert-butylate; hydrogen In tetrahydrofuran at 50℃; under 15001.5 Torr; for 4h; Glovebox; Autoclave; Sealed tube; chemoselective reaction; | 92% |
| With sodium tetrahydroborate In tetrahydrofuran at 25℃; for 4h; | 40% |
| With sodium tetrahydroborate In methanol for 5h; Cooling; | 19.09 g |
| With [MnBr(CO)3(2-(diphenylphosphinoamino)pyridine)]; potassium tert-butylate; hydrogen In ethanol at 80℃; for 48h; Autoclave; | 50.7 mg |
-
-
1444866-70-9
methyl benzylpyridin-2-ylcarbamate

-
-
6935-27-9
N-benzylpyridin-2-amine

| Conditions | Yield |
|---|---|
| With water; sodium hydroxide In methanol for 3h; Reflux; | 98% |
-
-
442513-39-5
benzyl-pyridin-2-yl-carbamic acid tert-butyl ester

-
-
6935-27-9
N-benzylpyridin-2-amine

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In dichloromethane; water | 97% |
| With trifluoroacetic acid In dichloromethane; water at 25℃; |

| Conditions | Yield |
|---|---|
| With dibenzylphosphoramidous acid 1,1'-binaphthyl-2,2'-diyl ester; caesium carbonate; copper(I) bromide In N,N-dimethyl-formamide at 90℃; for 36h; | 97% |
| With copper(l) iodide; 1-tetralone oxime; potassium hydroxide In water at 65℃; for 18h; Sealed tube; Inert atmosphere; Green chemistry; | 95% |
| With cesium acetate; copper In dimethyl sulfoxide at 90℃; for 24h; Inert atmosphere; | 92% |
-
-
1335111-43-7
N-benzyl-N-(pyridin-2-yl)hydroxylamine

-
-
6935-27-9
N-benzylpyridin-2-amine

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In methanol at 20℃; under 760.051 Torr; for 0.5h; Inert atmosphere; | 97% |

| Conditions | Yield |
|---|---|
| With [RuCl(CO)(PPh3)(PNS-Me)]; potassium hydroxide In toluene at 100℃; for 12h; | 96% |
| With bis(5-chlorosalicylidene)hydrazone binuclear copper(I) complex; potassium hydroxide In toluene at 100℃; for 18h; | 80% |
| With aluminum (III) chloride; water In acetonitrile at 20℃; Irradiation; | 50% |

| Conditions | Yield |
|---|---|
| With diethyl 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate; silver trifluoromethanesulfonate In dichloromethane at 20℃; for 2h; | 93% |
| With diethyl 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate In neat (no solvent) at 150℃; for 0.25h; | 92% |
| With diethyl 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate; thiourea In toluene at 70℃; for 24h; | 91% |
-
-
504-29-0
2-aminopyridine

-
-
873-76-7
para-Chlorobenzyl alcohol

-
A
-
6935-27-9
N-benzylpyridin-2-amine

-
B
-
22881-33-0
N-[(4-chlorophenyl)methyl]pyridin-2-amine

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; copper diacetate In 1,4-dioxane at 130℃; for 24h; | A 8% B 90% |
| With cesium hydroxide; palladium diacetate In toluene at 150℃; for 12h; | A 19% B 74% |

-
-
4931-08-2
N-(pyridin-2-yl)benzimidamide

-
-
100-51-6
benzyl alcohol

-
A
-
6935-27-9
N-benzylpyridin-2-amine

-
B
-
55-21-0
benzamide

| Conditions | Yield |
|---|---|
| With potassium hydroxide In toluene at 120℃; for 15h; Schlenk technique; Glovebox; Inert atmosphere; Sealed tube; | A 82% B 94 %Chromat. |


-
-
6935-27-9
N-benzylpyridin-2-amine

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 0 - 60℃; for 3h; Julia-Kocienski Olefination; | 80% |
-
-
78508-26-6
2-<α-(p-tolylthio)benzylamino>pyridine

-
-
6935-27-9
N-benzylpyridin-2-amine

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In 1,2-dimethoxyethane for 1h; Heating; | 75% |

-
-
6935-27-9
N-benzylpyridin-2-amine

| Conditions | Yield |
|---|---|
| With acetic acid; zinc at 20℃; for 29h; Reduction; | 75% |
-
-
504-29-0
2-aminopyridine

-
-
109-73-9
N-butylamine

-
-
100-51-6
benzyl alcohol

-
A
-
6935-27-9
N-benzylpyridin-2-amine

-
B
-
1077-18-5
N-benzylidenebutan-1-amine

| Conditions | Yield |
|---|---|
| With iron(II,III) oxide; potassium tert-butylate In 1,4-dioxane at 90℃; for 168h; Inert atmosphere; | A 74% B 26% |


| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; bromo-tris(1-pyrrolidinyl)phosphonium hexafluorophosphate In dichloromethane at 25℃; for 15h; | 70% |

| Conditions | Yield |
|---|---|
| With oxygen; lithium carbonate; copper(II) bis(trifluoromethanesulfonate) In toluene at 100℃; under 760.051 Torr; for 12h; Schlenk technique; | 62% |
| With sodium hydrogencarbonate In tetrahydrofuran at 20℃; Inert atmosphere; | 56% |
-
-
100-46-9
benzylamine

-
-
202923-80-6
pyridin-2-yl 1,1,2,2,3,3,4,4,4-nonafluorobutane-1-sulfonate

-
-
6935-27-9
N-benzylpyridin-2-amine

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide at 100℃; for 24h; | 60% |
-
-
504-29-0
2-aminopyridine

-
-
100-46-9
benzylamine

-
A
-
6935-27-9
N-benzylpyridin-2-amine

-
B
-
620-40-6
tribenzylamine

-
C
-
103-49-1
dibenzylamine

| Conditions | Yield |
|---|---|
| With C4F9SO3H at 200℃; for 42h; Yields of byproduct given; | A 54% B n/a C n/a |


| Conditions | Yield |
|---|---|
| With di-tert-butyl peroxide; copper In neat (no solvent) at 120℃; for 12h; Inert atmosphere; Schlenk technique; | 51% |

| Conditions | Yield |
|---|---|
| With cesium acetate; copper In dimethyl sulfoxide at 110℃; for 24h; Inert atmosphere; | 50% |
| In 1-methyl-pyrrolidin-2-one at 150℃; for 0.25h; Flow reactor; |
-
-
504-29-0
2-aminopyridine

-
-
100-44-7
benzyl chloride

-
A
-
6935-27-9
N-benzylpyridin-2-amine

-
B
-
137002-80-3
2-Benzylamino-5-benzylpyridine

| Conditions | Yield |
|---|---|
| 1) 180 to 250 deg C, 3 h, 2) 250 deg C, 0.5 h; | A 20% B 41% |

-
-
5683-33-0
2-(Dimethylamino)pyridine

-
-
100-44-7
benzyl chloride

-
A
-
6935-27-9
N-benzylpyridin-2-amine

-
B
-
20173-75-5
N-benzyl-N-methyl-N-(2-pyridyl)amine

-
C
-
137002-80-3
2-Benzylamino-5-benzylpyridine

-
D
-
154424-43-8
2-(N,N-dibenzylamino)pyridine

| Conditions | Yield |
|---|---|
| at 250℃; for 4h; | A 15% B 21% C 4.7% D 19% |

-
-
100-44-7
benzyl chloride

-
A
-
6935-27-9
N-benzylpyridin-2-amine

-
B
-
98477-40-8
2-amino-5-benzylpyridine

-
C
-
20173-75-5
N-benzyl-N-methyl-N-(2-pyridyl)amine

-
D
-
154424-43-8
2-(N,N-dibenzylamino)pyridine

| Conditions | Yield |
|---|---|
| at 250℃; for 4h; Further byproducts given; | A 17% B 4.3% C 20% D 14% |

-
-
100-44-7
benzyl chloride

-
A
-
6935-27-9
N-benzylpyridin-2-amine

-
B
-
20173-75-5
N-benzyl-N-methyl-N-(2-pyridyl)amine

-
C
-
137002-80-3
2-Benzylamino-5-benzylpyridine

-
D
-
154424-43-8
2-(N,N-dibenzylamino)pyridine

| Conditions | Yield |
|---|---|
| at 250℃; for 4h; Further byproducts given; | A 17% B 20% C 14% D 12% |

-
-
504-29-0
2-aminopyridine

-
-
120-51-4
benzoic acid benzyl ester

-
A
-
1753-62-4
N-benzyl-2-pyridone

-
B
-
6935-27-9
N-benzylpyridin-2-amine

-
C
-
55-21-0
benzamide

-
D
-
65-85-0
benzoic acid

| Conditions | Yield |
|---|---|
| at 190 - 200℃; anschliessend Behandeln mit wss. Salzsaeure; |

-
-
504-29-0
2-aminopyridine

-
-
64-18-6
formic acid

-
-
100-52-7
benzaldehyde

-
-
6935-27-9
N-benzylpyridin-2-amine

-
-
12112-67-3
bis(1,5-cyclooctadiene)diiridium(I) dichloride

-
-
6935-27-9
N-benzylpyridin-2-amine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 7h; | 100% |
-
-
6935-27-9
N-benzylpyridin-2-amine

| Conditions | Yield |
|---|---|
| In neat (no solvent) stirring at 120°C, 16h; sublimating excess ligand; repeating heating with fresh benzylaminopyridine; collecting; washing (acetone, methanol, diethyl ether); drying (vac., 80°C); elem. anal.; | 97% |

| Conditions | Yield |
|---|---|
| With 1,1,1,3',3',3'-hexafluoro-propanol; bis(tertbutylcarbonyloxy)iodobenzene at 25℃; for 7h; Reagent/catalyst; Solvent; | 97% |

| Conditions | Yield |
|---|---|
| Stage #1: N-benzylpyridin-2-amine With methylmagnesium chloride In tetrahydrofuran at 20℃; for 0.166667h; Stage #2: acetyl chloride In tetrahydrofuran at 20℃; for 1h; | 94% |
-
-
6935-27-9
N-benzylpyridin-2-amine

| Conditions | Yield |
|---|---|
| In chlorobenzene for 3h; Reflux; | 93% |
-
-
6935-27-9
N-benzylpyridin-2-amine

-
-
2938-91-2
(butane-1-sulfenyl)-dipropyl-borane

-
-
64738-97-2
(C3H7)2BN(CH2C6H5)(2-C5H4N)

| Conditions | Yield |
|---|---|
| In dichloromethane byproducts: C4H9SH; | 92% |
| In dichloromethane byproducts: C4H9SH; | 92% |
-
-
6935-27-9
N-benzylpyridin-2-amine

-
-
280116-83-8
benzyl(5-bromopyridin-2-yl)amine

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; 1-butylpyridinium bromide; toluene-4-sulfonic acid In 1,2-dimethoxyethane at 80℃; for 24h; Reagent/catalyst; Schlenk technique; Inert atmosphere; Green chemistry; regioselective reaction; | 91% |
-
-
6935-27-9
N-benzylpyridin-2-amine

-
-
15781-72-3
bis(2,4,6-trichlorophenyl) ethylmalonate

| Conditions | Yield |
|---|---|
| at 180℃; for 0.0833333h; Cyclization; | 86% |
| at 160℃; for 0.0666667h; Condensation; | 70% |
-
-
50-00-0
formaldehyd

-
-
6935-27-9
N-benzylpyridin-2-amine

-
-
829-85-6
diphenylphosphane

-
-
562108-07-0
2-(N-diphenylphosphinomethyl-N-benzyl)aminopyridine

| Conditions | Yield |
|---|---|
| With acetic acid In toluene at 70 - 80℃; for 6h; | 86% |


| Conditions | Yield |
|---|---|
| With sulfuric acid for 24h; Ambient temperature; | 85% |
| With 9,10-Dicyanoanthracene In water; acetonitrile Irradiation; | 75% |
-
-
6935-27-9
N-benzylpyridin-2-amine

-
-
100-44-7
benzyl chloride

-
-
154424-43-8
2-(N,N-dibenzylamino)pyridine

| Conditions | Yield |
|---|---|
| With sodium amide In toluene | 83% |
-
-
6935-27-9
N-benzylpyridin-2-amine

-
-
57072-87-4
chlorocarbonyl-diazo-acetic acid ethyl ester

| Conditions | Yield |
|---|---|
| 83% |
-
-
6935-27-9
N-benzylpyridin-2-amine

-
A
-
245-47-6
benzo[4,5]imidazo[1,2-a]pyridine

-
B
-
1445436-82-7
8-methyl-4-chloropyrido[1,2-a]benzimidazole

| Conditions | Yield |
|---|---|
| With 1,1,1,3',3',3'-hexafluoro-propanol; bis(tertbutylcarbonyloxy)iodobenzene at 25℃; for 7h; | A 83% B 79% |

-
-
6935-27-9
N-benzylpyridin-2-amine

-
-
304857-39-4
(6R,8R)-(-)-2-chloro-5,6,7,8,-tetrahydro-7,7-dimethyl-[6,8-methanoquinoline]

| Conditions | Yield |
|---|---|
| With 1,3-bis-(diphenylphosphino)propane; bis(dibenzylideneacetone)-palladium(0); sodium t-butanolate; tetrabutylammomium bromide In toluene at 100℃; Buchwald-Hartwig amination reaction; | 81% |
-
-
6935-27-9
N-benzylpyridin-2-amine

-
-
536-74-3
phenylacetylene

-
-
61122-85-8
phenyl-(2-phenylimidazo[1,2-a]pyridin-3-yl)methanone

| Conditions | Yield |
|---|---|
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; oxygen; lithium carbonate; copper(II) bis(trifluoromethanesulfonate) In toluene at 100℃; under 760.051 Torr; for 12h; Schlenk technique; | 81% |
-
-
6935-27-9
N-benzylpyridin-2-amine

-
-
3282-30-2
pivaloyl chloride

-
-
521300-02-7
N-benzyl-N-(pyridin-2-yl)pivalamide

| Conditions | Yield |
|---|---|
| Stage #1: N-benzylpyridin-2-amine With methylmagnesium chloride In tetrahydrofuran at 20℃; for 0.166667h; Stage #2: pivaloyl chloride In tetrahydrofuran at 20℃; for 1h; | 79% |
2-benzylaminopyridine Chemical Properties
IUPAC Name: N-benzylpyridin-2-amine
Molecular Formula: C12H12N2
Molecular Weight: 184.2371g/mol
Appearance: white to off-white powder
EINECS: 230-060-5
Density: 1.131 g/cm3
Melting Point: 94°C to 98°C
Boiling Point: 116-131°C(0.6 mmHg),321.8 °C at 760 mmHg
Flash Point: 148.4 °C
Freely Rotating Bonds: 2
Polar Surface Area: 16.13 Å2
Index of Refraction: 1.636
Molar Refractivity: 58.44 cm3
Molar Volume: 162.8 cm3
Polarizability: 23.16 ×10-24cm3
Surface Tension: 49.7 dyne/cm
Enthalpy of Vaporization: 56.36 kJ/mol
Vapour Pressure: 0.000291 mmHg at 25°C
The chemical synonyms of 2-Benzylaminopyridine (6935-27-9) are Benzyl-pyridin-2-yl-amine ; Aurora ka-4160 ; Alpha-benzylaminopyridine ; 2-Benzylaminopyridine ; n-Benzyl-2-pyridylamine ; n-Benzylpyridin-2-amine ; n-(2-pyridyl)Benzylamine ; n-(Phenylmethyl)-2-pyridinamine .Product categories of 2-Benzylaminopyridine (6935-27-9) are Pyridines derivates ; C9 to C46 ; Heterocyclic Building Blocks and Pyridines .The molecular structure of 2-Benzylaminopyridine (6935-27-9) is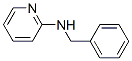 .
.
2-benzylaminopyridine Uses
It can be used as pharmaceutical intermediate.
2-benzylaminopyridine Safety Profile
Hazard Codes: .gif) Xn,Xi
Xn,Xi
Xn: Harmful
Xi: Irritant
Risk Statements: 22-37/38-41-40-36/37/38-20/21/22
22: Harmful if swallowed
40: Limited evidence of a carcinogenic effect
41: Risk of serious damage to eyes
20/21/22: Harmful by inhalation, in contact with skin and if swallowed
37/38: Irritating to respiratory system and skin
36/37/38: Irritating to eyes, respiratory system and skin
Safety Statements; 26-39-36-22
22: Do not breathe dust
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36: Wear suitable protective clothing
39: Wear eye/face protection
Related Products
- 2-benzylaminopyridine
- 2-BENZYLAMINOPYRIDINE HYDROCHLORIDE
- 69353-21-5
- 6935-39-3
- 6935-44-0
- 693-54-9
- 693-55-0
- 69355-99-3
- 693-57-2
- 693-58-3
- 69360-26-5
- 69363-09-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View