-
Name
2-HYDROXYTETRAHYDROPYRAN
- EINECS 229-509-8
- CAS No. 694-54-2
- Article Data104
- CAS DataBase
- Density 1.08 g/cm3
- Solubility
- Melting Point
- Formula C5H10O2
- Boiling Point 170.5 °C at 760 mmHg
- Molecular Weight 102.133
- Flash Point 71.4 °C
- Transport Information
- Appearance
- Safety 36/37/39-26
- Risk Codes 36/37/38-20/22
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Pyran-2-ol,tetrahydro- (6CI,7CI);2-Hydroxytetrahydro-2H-pyran;2-Hydroxytetrahydropyran;NSC 244915;Tetrahydro-2-hydroxy-2H-pyran;Tetrahydro-2H-pyran-2-ol;Tetrahydropyran-2-ol;d-Valerolactol;
- PSA 29.46000
- LogP 0.50530
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water at 0 - 20℃; for 1.25h; Inert atmosphere; | 96% |
| With hydrogenchloride In water at 0 - 20℃; for 1.5h; | 93% |
| With hydrogenchloride; water In water at 0 - 20℃; for 1.25h; | 87% |

-
-
694-54-2
tetrahydro-2H-2-pyranol

| Conditions | Yield |
|---|---|
| With methanol; iodine; magnesium at 40℃; for 3h; | 96% |

| Conditions | Yield |
|---|---|
| With diisobutylaluminium hydride In diethyl ether; hexane at -78℃; for 2h; Reduction; | 93% |
| With diisobutylaluminium hydride In hexane; dichloromethane at -78℃; for 2h; | 91% |
| With diisobutylaluminium hydride In hexane; dichloromethane at -78℃; for 0.25h; | 88% |
-
-
110-87-2
3,4-dihydro-2H-pyran

-
-
629-11-8
1,6-hexanediol

-
A
-
694-54-2
tetrahydro-2H-2-pyranol

-
B
-
28659-22-5
6-(tetrahydro-2H-pyranyloxy)hexan-1-ol

-
C
-
15057-15-5
1,6-bis(tetrahydropyranyloxy)hexane

| Conditions | Yield |
|---|---|
| With Dowex 50W x 2 In toluene at 30℃; for 2.5h; Etherification; hydration; | A n/a B 89% C 2% |

-
-
157136-52-2
ethyl 2-<1-(2-tetrahydropyranylperoxy)ethyl>propenoate

-
A
-
694-54-2
tetrahydro-2H-2-pyranol

-
B
-
592-84-7
n-butyl formate

| Conditions | Yield |
|---|---|
| With tert-butyl peroxyacetate In cyclohexane at 110℃; under 0.001 Torr; for 12h; further solvents; | A 1 % Chromat. B 6 % Chromat. C 7% D 75% |

-
-
258331-72-5
5-triphenylmethoxypentanal

-
-
694-54-2
tetrahydro-2H-2-pyranol

| Conditions | Yield |
|---|---|
| Stage #1: 5-triphenylmethoxypentanal With boron trichloride In dichloromethane at -30℃; for 0.75h; Substitution; Stage #2: With methanol Cyclization; methanolysis; Further stages.; | 75% |
-
-
25073-26-1
(E)-pent-2-ene-1,5-diol

-
-
201230-82-2
carbon monoxide

-
A
-
694-54-2
tetrahydro-2H-2-pyranol

-
B
-
111-29-5
1 ,5-pentanediol

-
C
-
123703-40-2
2,3,3aβ,4,5,6aβ-perhydrofuro<2,3b>furan

| Conditions | Yield |
|---|---|
| With chloro(1,5-cyclooctadiene)rhodium(I) dimer; hydrogen; triphenylphosphine In dichloromethane at 120℃; under 45003.6 Torr; for 20h; Product distribution; Further Variations:; Reagents; Solvents; | A n/a B n/a C 72% D n/a |

-
-
25073-26-1
(E)-pent-2-ene-1,5-diol

-
-
201230-82-2
carbon monoxide

-
A
-
694-54-2
tetrahydro-2H-2-pyranol

-
B
-
123703-40-2
2,3,3aβ,4,5,6aβ-perhydrofuro<2,3b>furan

| Conditions | Yield |
|---|---|
| With chloro(1,5-cyclooctadiene)rhodium(I) dimer; hydrogen; triphenylphosphine In dichloromethane at 120℃; under 45003.6 Torr; for 20h; | A n/a B 72% |


| Conditions | Yield |
|---|---|
| With copper(l) iodide; zinc In water ultrasonic irradiation; | 70% |
-
-
542-28-9
3,4,5,6-tetrahydro-2H-pyran-2-one

-
A
-
694-54-2
tetrahydro-2H-2-pyranol

-
B
-
4221-03-8
5-hydroxypentanal

| Conditions | Yield |
|---|---|
| With diisobutylaluminium hydride In dichloromethane; toluene at -70℃; for 3h; Inert atmosphere; Overall yield = 0.71 g; | A 65% B 25% |
-
-
111-29-5
1 ,5-pentanediol

-
A
-
694-54-2
tetrahydro-2H-2-pyranol

-
B
-
542-28-9
3,4,5,6-tetrahydro-2H-pyran-2-one

| Conditions | Yield |
|---|---|
| With 1-methyl-1H-imidazole; [2,2]bipyridinyl; tetrakis(acetonitrile)copper(I) trifluoromethanesulfonate; 9-azabicyclo<3.3.1>nonane-N-oxyl In acetonitrile at 22℃; for 2h; | A 58% B 29% |
-
-
69161-61-1, 4203-49-0
2-(prop-2-en-1-yloxy)oxane

-
-
75-24-1
trimethylaluminum

-
A
-
694-54-2
tetrahydro-2H-2-pyranol

| Conditions | Yield |
|---|---|
| bis(triphenylphosphine)nickel(II) chloride In toluene | A n/a B 48% |

-
-
29293-07-0
pent-2-ene-1,5-diol

-
-
201230-82-2
carbon monoxide

-
A
-
694-54-2
tetrahydro-2H-2-pyranol

-
B
-
123703-40-2
2,3,3aβ,4,5,6aβ-perhydrofuro<2,3b>furan

| Conditions | Yield |
|---|---|
| With hydrogen; triphenylphosphine; chloro(1,5-cyclooctadiene)rhodium(I) dimer In 1,4-dioxane at 120℃; under 45004.5 Torr; for 20h; | A 7% B 35% C 34% |


| Conditions | Yield |
|---|---|
| platinum In sulfuric acid; water at 35℃; for 1.15h; Product distribution; electrolysis, also with PtO2, RuO2; | 91 % Turnov. |
| platinum In sulfuric acid; water at 35℃; for 1.15h; electrolysis; | 91 % Turnov. |
-
-
142-68-7
TETRAHYDROPYRANE

-
A
-
694-54-2
tetrahydro-2H-2-pyranol

-
B
-
542-28-9
3,4,5,6-tetrahydro-2H-pyran-2-one

| Conditions | Yield |
|---|---|
| With 1,1,1-trifluoro-2-propanone; methyltrifluoromethyldioxirane In dichloromethane at 0℃; for 0.25h; Yield given. Yields of byproduct given; | |
| lead dioxide In sulfuric acid; water at 35℃; for 7.83333h; electrolysis; | A 5.4 % Turnov. B 73 % Turnov. |
| With oxone; methyltrifluoromethyldioxirane In dichloromethane at -15℃; | A 37 % Chromat. B 63 % Chromat. |
-
-
110-87-2
3,4-dihydro-2H-pyran

-
A
-
694-54-2
tetrahydro-2H-2-pyranol

-
B
-
709-84-2
bis-tetrahydropyran-2-yl ether

-
C
-
14194-86-6
5-(2-tetrahydropyranyloxy)pentanal

-
D
-
4221-03-8
5-hydroxypentanal

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 0.25h; Product distribution; Ambient temperature; other times and temperatures, other hydration reagent; | |
| With hydrogenchloride; sodium sulfite at 25℃; for 24h; Yield given; | |
| With hydrogenchloride at 25℃; for 0.25h; Yield given. Yields of byproduct given; |

-
-
110-87-2
3,4-dihydro-2H-pyran

-
A
-
694-54-2
tetrahydro-2H-2-pyranol

-
B
-
709-84-2
bis-tetrahydropyran-2-yl ether

-
C
-
4221-03-8
5-hydroxypentanal

| Conditions | Yield |
|---|---|
| With hydrogenchloride 1.) 25 deg C, 1 h, 2.) reflux, 24 h; Yield given. Yields of byproduct given; |

-
-
110-87-2
3,4-dihydro-2H-pyran

-
A
-
694-54-2
tetrahydro-2H-2-pyranol

-
B
-
14194-86-6
5-(2-tetrahydropyranyloxy)pentanal

-
C
-
4221-03-8
5-hydroxypentanal

| Conditions | Yield |
|---|---|
| With sodium metabisulfite; water at 25℃; for 24h; Yield given. Yields of byproduct given; |

-
-
110-94-1
1,5-pentanedioic acid

-
A
-
694-54-2
tetrahydro-2H-2-pyranol

-
B
-
542-28-9
3,4,5,6-tetrahydro-2H-pyran-2-one

-
C
-
111-29-5
1 ,5-pentanediol

| Conditions | Yield |
|---|---|
| With H4Ru4(CO)84; hydrogen In diethyl ether; toluene at 200℃; under 98800 Torr; for 48h; | A 2.9 % Chromat. B 28.7 % Chromat. C 47.7 % Chromat. D 20.7 % Chromat. |

-
-
4203-50-3
2-phenoxytetrahydropyran

-
-
111-87-5
octanol

-
A
-
694-54-2
tetrahydro-2H-2-pyranol

-
B
-
70690-19-6
2-octyloxy-tetrahydro-pyran

-
C
-
108-95-2
phenol

| Conditions | Yield |
|---|---|
| tris(2,2'-bipyridyl)ruthenium dichloride; Paraquat In acetonitrile at 20℃; for 30h; Product distribution; Irradiation; also with H2O, or molecular sieves 4 Angstroem; |

-
-
4203-50-3
2-phenoxytetrahydropyran

-
A
-
694-54-2
tetrahydro-2H-2-pyranol

-
B
-
70690-19-6
2-octyloxy-tetrahydro-pyran

-
C
-
108-95-2
phenol

| Conditions | Yield |
|---|---|
| With water; tris(2,2'-bipyridyl)ruthenium dichloride; (MV)Cl2 In acetonitrile for 30h; Irradiation; Yields of byproduct given; |

-
-
69556-90-7, 77213-02-6
C5H9O2(1-)

-
-
694-54-2
tetrahydro-2H-2-pyranol

| Conditions | Yield |
|---|---|
| Thermodynamic data; protonation, ΔG0; |
-
-
66-25-1
hexanal

-
-
139048-07-0
2-Acetyl-2-methyl-5-oxo-hexanoic acid allyl ester

-
A
-
694-54-2
tetrahydro-2H-2-pyranol

-
B
-
139025-72-2, 139025-73-3
3-(1-Hydroxy-hexyl)-3-methyl-heptane-2,6-dione

| Conditions | Yield |
|---|---|
| With bis(triphenylphosphine)nickel(II) chloride; zinc In N,N-dimethyl-formamide at 40℃; for 5h; Yield given. Title compound not separated from byproducts; |


| Conditions | Yield |
|---|---|
| With acetylacetonatodicarbonylrhodium(l); (R,S)-binaphos; hydrogen In benzene at 60℃; under 22800 Torr; Yield given. Yields of byproduct given; | |
| With acetylacetonatodicarbonylrhodium(l); (R,S)-binaphos; hydrogen In benzene at 60℃; under 22800 Torr; Yield given. Yields of byproduct given. Title compound not separated from byproducts; |

-
-
69161-61-1, 4203-49-0
2-(prop-2-en-1-yloxy)oxane

-
-
694-54-2
tetrahydro-2H-2-pyranol

| Conditions | Yield |
|---|---|
| With triethylaluminum; bis(triphenylphosphine)nickel(II) chloride In toluene |
-
-
74266-26-5
α-bromopropionate de tetrahydropyrannyle

-
-
694-54-2
tetrahydro-2H-2-pyranol

| Conditions | Yield |
|---|---|
| With sodium hydroxide; potassium dihydrogenphosphate In water at 15℃; Rate constant; Mechanism; |

-
-
694-54-2
tetrahydro-2H-2-pyranol

| Conditions | Yield |
|---|---|
| With sodium hydroxide; potassium dihydrogenphosphate In water at 15℃; Rate constant; Mechanism; |

-
-
694-54-2
tetrahydro-2H-2-pyranol

| Conditions | Yield |
|---|---|
| With sodium hydroxide; potassium dihydrogenphosphate In water at 15℃; Rate constant; Mechanism; |

-
-
694-54-2
tetrahydro-2H-2-pyranol

| Conditions | Yield |
|---|---|
| With sodium hydroxide; potassium dihydrogenphosphate In water at 15℃; Rate constant; Mechanism; |

-
-
694-54-2
tetrahydro-2H-2-pyranol

| Conditions | Yield |
|---|---|
| With sodium hydroxide; potassium dihydrogenphosphate In water at 15℃; Rate constant; Mechanism; |

| Conditions | Yield |
|---|---|
| With triethyl borane; bis(acetylacetonate)nickel(II) In tetrahydrofuran; hexane at 20℃; for 40h; | 99% |
-
-
694-54-2
tetrahydro-2H-2-pyranol

-
-
18113-18-3
2,5-dimethoxyphenol

-
-
109765-65-3
2,5-Dimethoxy-1-(tetrahydro-2H-pyran-2-yloxy)-benzol

| Conditions | Yield |
|---|---|
| With tributylphosphine; 1,1'-azodicarbonyl-dipiperidine In tetrahydrofuran at 0 - 20℃; for 18h; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With 2-Sulfanylpyridine; FeIIFeIII2(μ3-O)(μ2-OOCCF3)6(OH2)3 In 1,2-dichloro-ethane at 60℃; for 24h; Reagent/catalyst; | 99% |
-
-
694-54-2
tetrahydro-2H-2-pyranol

-
-
55362-80-6
9-bromononan-1-ol

-
-
55695-90-4
2-(9-bromononyloxy)tetrahydropyran

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In tetrahydrofuran at 20℃; | 99% |
-
-
694-54-2
tetrahydro-2H-2-pyranol

-
-
68731-27-1
(3-bromopropyl)(methyl)sulfane

| Conditions | Yield |
|---|---|
| Stage #1: (3-bromopropyl)(methyl)sulfane With iodine; magnesium In tetrahydrofuran Reflux; Inert atmosphere; Stage #2: tetrahydro-2H-2-pyranol In tetrahydrofuran at 20℃; for 2h; Inert atmosphere; | 99% |
-
-
694-54-2
tetrahydro-2H-2-pyranol

-
-
21382-82-1
(carbethoxyethylidene)triphenylphosphorane

-
-
74844-86-3
(E)-7-hydroxy-2-methyl-hept-2-enoic acid ethyl ester

| Conditions | Yield |
|---|---|
| 98% | |
| In benzene for 12h; Ambient temperature; | 80% |
| In toluene at 90℃; for 1h; | |
| In benzene Wittig Olefination; | 1.87 g |

| Conditions | Yield |
|---|---|
| With carbon tetrabromide; triphenylphosphine at 20℃; for 16h; | 97% |
| With titanium(IV) tetrabutoxide; (R)-Mandelic Acid at 20℃; for 24h; | 94% |
-
-
694-54-2
tetrahydro-2H-2-pyranol

-
-
100-51-6
benzyl alcohol

-
-
1927-62-4
tetrahydro-2-(benzyloxy)-2H-pyran

| Conditions | Yield |
|---|---|
| With pyrrolidine hydrochloride In toluene at 100℃; for 5h; Inert atmosphere; | 96% |
-
-
694-54-2
tetrahydro-2H-2-pyranol

-
-
41879-39-4
O-(tert-butyldimethylsilanyl)hydroxylamine

-
-
873692-60-5
5-hydroxypentanal O-tert-butyldimethylsilyloxime

| Conditions | Yield |
|---|---|
| With magnesium sulfate In diethyl ether at 20℃; for 1h; | 94% |
-
-
694-54-2
tetrahydro-2H-2-pyranol

-
-
110-87-2
3,4-dihydro-2H-pyran

-
-
709-84-2
bis-tetrahydropyran-2-yl ether

| Conditions | Yield |
|---|---|
| With N.N'-bis[3,5-bis(trifluoromethyl)phenyl]thiourea at 20℃; for 19h; | 94% |
-
-
694-54-2
tetrahydro-2H-2-pyranol

-
-
1439-36-7
1-triphenylphosphoranylidene-2-propanone

-
-
172877-31-5
(E)-8-Hydroxy-oct-3-en-2-one

| Conditions | Yield |
|---|---|
| 93% |
-
-
694-54-2
tetrahydro-2H-2-pyranol

-
-
7103-09-5
4-but-1-enylmagnesium bromide

-
-
198637-27-3
non-8-ene-1,5-diol

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 1.5h; Ambient temperature; | 93% |

| Conditions | Yield |
|---|---|
| With 1-methyl-pyrrolidin-2-one; Li2CuCl4 In tetrahydrofuran at 20℃; for 1h; Inert atmosphere; Schlenk technique; | 93% |


| Conditions | Yield |
|---|---|
| With ammonium hydroxide; hydrogen In water at 80℃; under 15001.5 Torr; for 6h; Pressure; Temperature; | 93% |
| With ammonia; hydrogen In water at 80℃; under 15001.5 Torr; for 1h; Reagent/catalyst; Autoclave; | |
| With ammonium hydroxide; hydrogen In water at 60℃; under 15001.5 Torr; for 1h; Catalytic behavior; Temperature; Autoclave; |
-
-
694-54-2
tetrahydro-2H-2-pyranol

-
-
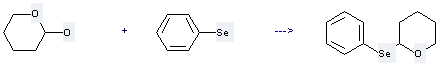
645-96-5
Benzeneselenol

-
-
64042-26-8
tetrahydro-2-(phenylseleno)-2H-Pyran

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In benzene at 25℃; | 92% |
-
-
694-54-2
tetrahydro-2H-2-pyranol

-
-
1100-88-5
benzyltriphenylphosphonium chloride

-
-
98078-15-0
6-phenylhex-5-en-1-ol

| Conditions | Yield |
|---|---|
| Stage #1: benzyltriphenylphosphonium chloride With potassium tert-butylate In tert-butyl alcohol at 20℃; for 0.5h; Stage #2: tetrahydro-2H-2-pyranol In tert-butyl alcohol at 20℃; for 0.5h; | 92% |

| Conditions | Yield |
|---|---|
| With (2S)-2-{diphenyl[(trimethylsilyl)oxy]methyl}pyrrolidine; benzoic acid In chloroform at 23℃; for 24h; Michael Addition; enantioselective reaction; | A 92% B n/a |
| Stage #1: tetrahydro-2H-2-pyranol; 2-(2-nitro-vinyl)-phenol With (2S)-2-{diphenyl[(trimethylsilyl)oxy]methyl}pyrrolidine; benzoic acid In chloroform at 25℃; for 24h; Stage #2: With hydrogenchloride In dichloromethane; water for 2h; Overall yield = 92 %; | A n/a B n/a |


| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; for 18h; Passerini Condensation; | 92% |


| Conditions | Yield |
|---|---|
| With benzoic acid In toluene at 110℃; for 24h; Inert atmosphere; | 90% |
-
-
694-54-2
tetrahydro-2H-2-pyranol

-
-
60669-22-9
n-undecyltriphenylphosphonium bromide

-
-
149011-67-6
5-(Z,E)-hexadecenol

| Conditions | Yield |
|---|---|
| Stage #1: n-undecyltriphenylphosphonium bromide With n-butyllithium In tetrahydrofuran; hexane at 0℃; for 1h; Stage #2: tetrahydro-2H-2-pyranol In tetrahydrofuran; hexane at 0 - 20℃; Wittig reaction; | 89% |
-
-
694-54-2
tetrahydro-2H-2-pyranol

-
-
60-12-8
2-phenylethanol

-
-
1927-61-3
2-(2-phenylethoxy)tetrahydro-2H-pyran

| Conditions | Yield |
|---|---|
| With rhenium(VII) oxide In dichloromethane at 20℃; Time; Inert atmosphere; | 89% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; for 18h; Passerini Condensation; | 89% |

-
-
694-54-2
tetrahydro-2H-2-pyranol

-
-
63922-69-0
(2-trimethylsilylethylidene)triphenylphosphorane

-
-
119554-49-3
7-(trimethylsilyl)-(E,Z)-5-hepten-1-ol

| Conditions | Yield |
|---|---|
| 88% | |
| In tetrahydrofuran -78 deg C to r.t.; | 80% |
-
-
694-54-2
tetrahydro-2H-2-pyranol

-
-
1099-45-2
ethyl (triphenylphosphoranylidene)acetate

-
-
96251-91-1, 105198-41-2, 110935-49-4
ethyl cis/trans-7-hydroxy-2-heptenoate

| Conditions | Yield |
|---|---|
| In dichloromethane for 1.5h; Wittig reaction; Heating; | 88% |
| 85% | |
| 79% |
-
-
694-54-2
tetrahydro-2H-2-pyranol

-
-
1099-45-2
ethyl (triphenylphosphoranylidene)acetate

-
-
96251-91-1
ethyl (E)-7-hydroxyhept-2-enoate

| Conditions | Yield |
|---|---|
| In tetrahydrofuran Wittig reaction; Heating; | 88% |
| In dichloromethane for 48h; Ambient temperature; | 83% |
| In dichloromethane at 23℃; for 96h; Inert atmosphere; | 80% |
-
-
694-54-2
tetrahydro-2H-2-pyranol

-
-
59555-69-0
2-methyl-5-(phenylsulfonyl)-2-pentene

-
-
116893-79-9, 116893-80-2
9-methyl 6-phenylsulphonyl 8-decen 1,5-diol

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide at 20℃; for 18h; | 87% |
2H-Pyran-2-ol,tetrahydro- Specification
The 2H-Pyran-2-ol,tetrahydro- is an organic compound with the formula C5H10O2. The IUPAC name of this chemical is Oxan-2-ol. With the CAS registry number 694-54-2, it is also named as 2-Tetrahydropyranol. Besides, it is clear colorless liquid, which should be stored in a cool, sealed, dry place.
Physical properties about 2H-Pyran-2-ol,tetrahydro- are: (1)ACD/LogP: -0.36; (2)ACD/LogD (pH 5.5): -0.36; (3)ACD/LogD (pH 7.4): -0.36; (4)ACD/BCF (pH 5.5): 1; (5)ACD/BCF (pH 7.4): 1; (6)ACD/KOC (pH 5.5): 15.15; (7)ACD/KOC (pH 7.4): 15.15; (8)#H bond acceptors: 2; (9)#H bond donors: 1; (10)#Freely Rotating Bonds: 1; (11)Polar Surface Area: 18.46 Å2; (12)Index of Refraction: 1.467; (13)Molar Refractivity: 26.25 cm3; (14)Molar Volume: 94.4 cm3; (15)Polarizability: 10.4×10-24 cm3; (16)Surface Tension: 36 dyne/cm; (17)Density: 1.08 g/cm3; (18)Flash Point: 71.4 °C; (19)Enthalpy of Vaporization: 47.37 kJ/mol; (20)Boiling Point: 170.5 °C at 760 mmHg; (21)Vapour Pressure: 0.471 mmHg at 25 °C.
Preparation: this chemical can be prepared by Tetrahydro-pyran-2-one. This reaction will need reagent diisobutylaluminum hydride (DIBAL-H) and solvent toluene. The reaction time is 1 hour with reaction temperature of -70 °C.

Uses of 2H-Pyran-2-ol,tetrahydro-: it can be used to produce 2-Phenylselanyl-tetrahydro-pyran at temperature of 25 °C. It will need reagent p-toluenesulfonic acid and solvent benzene. The yield is about 92%.
When you are using this chemical, please be cautious about it as the following:
It is harmful by inhalation and if swallowed. Besides, this chemical is irritating to eyes, respiratory system and skin. When you are using it, wear suitable protective clothing, gloves and eye/face protection. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)InChI: InChI=1/C5H10O2/c6-5-3-1-2-4-7-5/h5-6H,1-4H2
(2)InChIKey: CELWCAITJAEQNL-UHFFFAOYAE
(3)Std. InChI: InChI=1S/C5H10O2/c6-5-3-1-2-4-7-5/h5-6H,1-4H2
(4)Std. InChIKey: CELWCAITJAEQNL-UHFFFAOYSA-N
Related Products
- 2H-Pyran-2-ol, 3-chlorotetrahydro-
- 2H-Pyran-2-ol,tetrahydro-
- 6945-54-6
- 6945-64-8
- 6945-67-1
- 6945-68-2
- 69457-70-1
- 6945-87-5
- 6945-92-2
- 694-59-7
- 6946-01-6
- 6946-14-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View