-
Name
3-(2-Chloroethyl)-6,7,8,9-tetrahydro-9-hydroxy-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one
- EINECS 603-382-2
- CAS No. 130049-82-0
- Article Data25
- CAS DataBase
- Density 1.41 g/cm3
- Solubility
- Melting Point 100-102°C
- Formula C11H15O2N2Cl
- Boiling Point 389.3 °C at 760 mmHg
- Molecular Weight 242.705
- Flash Point 189.2 °C
- Transport Information
- Appearance pale-yellow solid
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 3-(2-Chloroethyl)-6,7,8,9-tetrahydro-9-hydroxy-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one;3-(2-Chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one;3-(2-Chloroethyl)-9-hydroxy-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one;9-Hydroxy-3-(2-chloroethyl)-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one;
- PSA 55.12000
- LogP 1.16020
Synthetic route
-
-
260273-82-3
3-(2-chloroethyl)-2-methyl-9-hydroxy-4H-pyrido[1,2-a]pyrimidin-4-one

-
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

| Conditions | Yield |
|---|---|
| Stage #1: 3-(2-chloroethyl)-2-methyl-9-hydroxy-4H-pyrido[1,2-a]pyrimidin-4-one With hydrogenchloride; palladium 10% on activated carbon In ethanol; water Green chemistry; Stage #2: With hydrogen In ethanol; water at 80℃; under 11251.1 Torr; Catalytic behavior; Solvent; Reagent/catalyst; Temperature; Pressure; Green chemistry; | 99.32% |
| Stage #1: 3-(2-chloroethyl)-2-methyl-9-hydroxy-4H-pyrido[1,2-a]pyrimidin-4-one With hydrogen; palladium 10% on activated carbon In acetic acid at 25 - 30℃; under 2206.72 Torr; Stage #2: With sodium hydroxide In dichloromethane; water pH=5.5 - 6.0; Product distribution / selectivity; | |
| With hydrogen; palladium 10% on activated carbon In acetic acid at 35℃; under 735.572 - 2206.72 Torr; Product distribution / selectivity; |
-
-
849727-62-4
3-(2-chloroethyl)-9-hydroxy-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one monohydrochloride

-
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

| Conditions | Yield |
|---|---|
| Stage #1: 3-(2-chloroethyl)-9-hydroxy-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one monohydrochloride With hydrogen; 5%-palladium/activated carbon In water; isopropyl alcohol at 55℃; under 760.051 Torr; Inert atmosphere; Stage #2: With potassium acetate In water Product distribution / selectivity; | 92% |
| Stage #1: 3-(2-chloroethyl)-9-hydroxy-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one monohydrochloride In methanol at 50 - 55℃; Stage #2: With hydrogen; palladium 10% on activated carbon In methanol at 50 - 55℃; for 8h; | 68.25% |
| Stage #1: 3-(2-chloroethyl)-9-hydroxy-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one monohydrochloride With pyrographite In methanol at 50 - 55℃; for 0.75h; Large scale; Stage #2: With palladium 10% on activated carbon; hydrogen In methanol; dichloromethane at 25 - 30℃; under 2172.08 - 2947.82 Torr; Large scale; | 39% |
-
-
1204248-71-4
C11H14ClN3O2

-
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

| Conditions | Yield |
|---|---|
| Stage #1: C11H14ClN3O2 With hydrogenchloride; titanium(III) chloride; water at 70℃; for 0.5h; Inert atmosphere; Stage #2: With sodium hydrogencarbonate In water | 87% |
-
-
147687-17-0
9-(benzyloxy)-3-(2-chloroethyl)-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one

-
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride; palladium 10% on activated carbon; hydrogen In methanol; water; tert-butyl alcohol at 25 - 30℃; under 775.743 Torr; for 16h; Reagent/catalyst; Solvent; Pressure; | 74.4% |
| With hydrogenchloride; hydrogen; palladium 10% on activated carbon In water at 25 - 30℃; | 69% |
| With cyclohexene; palladium 10% on activated carbon for 19h; Product distribution / selectivity; Heating / reflux; |
-
-
130049-79-5
9-benzyloxy-3-(2-chloro-ethyl)-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

-
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

| Conditions | Yield |
|---|---|
| Stage #1: 9-benzyloxy-3-(2-chloro-ethyl)-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one With hydrogenchloride; hydrogen; 5% palladium over charcoal In methanol at 27℃; for 36h; pH=3.0; Inert atmosphere; Stage #2: With sodium hydroxide In water at 5℃; pH=10 - 11; | 72.6% |
| With hydrogenchloride; hydrogen; palladium 10% on activated carbon In water at 25 - 30℃; under 735.572 - 1471.14 Torr; for 4 - 6h; | 69% |
-
-
1117803-76-5
3-(2-chloroethyl)-6,7,8,9-tetrahydro-9-acetyloxy-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one

-
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water |
-
-
1254173-48-2
3-(2-chloroethyl)-9-hydroxy-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one monohydrochloride

-
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

| Conditions | Yield |
|---|---|
| Stage #1: 3-(2-chloroethyl)-9-hydroxy-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one monohydrochloride With hydrogen; 1% Pd/C In acetic acid at 35℃; under 2206.72 - 2574.5 Torr; for 6h; Stage #2: With sodium hydroxide In water at 0℃; for 1h; pH=6; Product distribution / selectivity; |
-
-
147687-17-0
9-(benzyloxy)-3-(2-chloroethyl)-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one

-
A
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

-
B
-
260273-82-3
3-(2-chloroethyl)-2-methyl-9-hydroxy-4H-pyrido[1,2-a]pyrimidin-4-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride; hydrogen; 1% Pd/C In methanol; water at 25 - 30℃; under 735.572 - 1471.14 Torr; for 1h; Product distribution / selectivity; Autoclave; | A 28.8 %Chromat. B 62 %Chromat. |
-
-
147687-17-0
9-(benzyloxy)-3-(2-chloroethyl)-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one

-
A
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

-
B
-
849903-79-3
3-ethyl-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride; hydrogen; 1% Pd/C In methanol; water at 25 - 30℃; under 735.572 - 1471.14 Torr; for 3.5h; Product distribution / selectivity; Autoclave; | A 48.9 %Chromat. B 39 %Chromat. |
-
-
1228559-69-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one oxalate

-
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

| Conditions | Yield |
|---|---|
| With potassium acetate In water at 0 - 5℃; for 1h; pH=4.8; |
-
-
517-23-7
3-acetyl-2-oxo-4,5-dihydrofuran

-
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: toluene-4-sulfonic acid / xylene / 20.5 h / 25 - 142 °C 2: hydrogenchloride / isopropyl alcohol; methanol / 1 h / 60 - 65 °C / pH 1 - 3 3: hydrogen; zinc(II) chloride / palladium on carbon / methanol / 35 - 55 °C View Scheme | |
| Multi-step reaction with 3 steps 1: toluene-4-sulfonic acid / xylene / Reflux 2: thionyl chloride / N,N-dimethyl-formamide / 65 - 70 °C 3: hydrogen / palladium 10% on activated carbon / methanol / 23 - 30 °C / 1471.14 - 2206.72 Torr View Scheme | |
| Multi-step reaction with 2 steps 1.1: trichlorophosphate / toluene 1.2: 7 h / 90 - 95 °C 1.3: 1 h / 90 - 95 °C 2.1: hydrogen / palladium 10% on activated carbon / acetic acid / 35 °C / 735.57 - 2206.72 Torr View Scheme | |
| Multi-step reaction with 3 steps 1.1: toluene / 0.25 h 1.2: 20 h / 70 °C / Reflux 2.1: hydrogenchloride / toluene; water / 9 h / 65 - 70 °C 3.1: hydrogen; palladium on activated charcoal; acetic acid / 6 h / 35 °C / 2250.23 - 2625.26 Torr / Autoclave View Scheme | |
| Multi-step reaction with 2 steps 1.1: trichlorophosphate / toluene / 24 h / 30 - 40 °C / Large scale 1.2: 0 - 5 °C / Large scale 2.1: pyrographite / methanol / 0.75 h / 50 - 55 °C / Large scale 2.2: 25 - 30 °C / 2172.08 - 2947.82 Torr / Large scale View Scheme |
-
-
181525-38-2
9-hydroxy-3-hydroxyethyl-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one

-
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: hydrogenchloride / isopropyl alcohol; methanol / 1 h / 60 - 65 °C / pH 1 - 3 2: hydrogen; zinc(II) chloride / palladium on carbon / methanol / 35 - 55 °C View Scheme | |
| Multi-step reaction with 2 steps 1: thionyl chloride / N,N-dimethyl-formamide / 65 - 70 °C 2: hydrogen / palladium 10% on activated carbon / methanol / 23 - 30 °C / 1471.14 - 2206.72 Torr View Scheme |
-
-
16867-03-1
2-amino-3-hydroxypyridine

-
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: toluene-4-sulfonic acid / xylene / 20.5 h / 25 - 142 °C 2: hydrogenchloride / isopropyl alcohol; methanol / 1 h / 60 - 65 °C / pH 1 - 3 3: hydrogen; zinc(II) chloride / palladium on carbon / methanol / 35 - 55 °C View Scheme | |
| Multi-step reaction with 3 steps 1: toluene-4-sulfonic acid / xylene / Reflux 2: thionyl chloride / N,N-dimethyl-formamide / 65 - 70 °C 3: hydrogen / palladium 10% on activated carbon / methanol / 23 - 30 °C / 1471.14 - 2206.72 Torr View Scheme | |
| Multi-step reaction with 2 steps 1.1: trichlorophosphate / toluene / 24 h / 30 - 40 °C / Large scale 1.2: 0 - 5 °C / Large scale 2.1: pyrographite / methanol / 0.75 h / 50 - 55 °C / Large scale 2.2: 25 - 30 °C / 2172.08 - 2947.82 Torr / Large scale View Scheme | |
| Multi-step reaction with 4 steps 1: sodium hydroxide; tetrabutylammomium bromide / water / 6 h / 40 - 75 °C / Large scale 2: toluene-4-sulfonic acid / toluene / Reflux; Large scale 3: trichlorophosphate / 1,2-dimethoxyethane / 6 h / Reflux; Large scale 4: hydrogenchloride; palladium 10% on activated carbon; hydrogen / water; methanol; tert-butyl alcohol / 16 h / 25 - 30 °C / 775.74 Torr View Scheme |
-
-
517-23-7
3-acetyl-2-oxo-4,5-dihydrofuran

-
-
24016-03-3
2-amino-3-benzyloxypyridine

-
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride; hydrogen; trichlorophosphate In cyclohexane; acetic acid; toluene |
-
-
24016-03-3
2-amino-3-benzyloxypyridine

-
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: trichlorophosphate / toluene 1.2: 7 h / 90 - 95 °C 1.3: 1 h / 90 - 95 °C 2.1: hydrogen / palladium 10% on activated carbon / acetic acid / 35 °C / 735.57 - 2206.72 Torr View Scheme | |
| Multi-step reaction with 3 steps 1.1: toluene / 0.25 h 1.2: 20 h / 70 °C / Reflux 2.1: hydrogenchloride / toluene; water / 9 h / 65 - 70 °C 3.1: hydrogen; palladium on activated charcoal; acetic acid / 6 h / 35 °C / 2250.23 - 2625.26 Torr / Autoclave View Scheme | |
| Multi-step reaction with 3 steps 1: toluene-4-sulfonic acid / toluene / Reflux; Large scale 2: trichlorophosphate / 1,2-dimethoxyethane / 6 h / Reflux; Large scale 3: hydrogenchloride; palladium 10% on activated carbon; hydrogen / water; methanol; tert-butyl alcohol / 16 h / 25 - 30 °C / 775.74 Torr View Scheme |

-
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen; acetic acid at 35℃; under 2250.23 - 2625.26 Torr; for 6h; Time; Temperature; Autoclave; | 22 g |
-
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

-
-
138271-16-6
(Z)-(2,4-difluorophenyl) (4-piperidinyl)methanone, oxime monohydrochloride

| Conditions | Yield |
|---|---|
| With potassium iodide; sodium hydroxide In acetonitrile for 10h; Reflux; | 99.2% |
-
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

-
-
107-30-2
chloromethyl methyl ether

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 45℃; Reagent/catalyst; | 98% |
-
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

-
-
57-10-3
1-hexadecylcarboxylic acid

-
-
1415488-05-9
3-(2-chloroethyl)-6,7,8,9-tetrahydro-2-methyl-9-hydroxy-4H-pyrido[1,2-a]pyrimidine-4-one palmitate ester

| Conditions | Yield |
|---|---|
| Stage #1: 3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one; 1-hexadecylcarboxylic acid With dmap; triethylamine In dichloromethane at 25 - 30℃; for 0.25h; Stage #2: With pivaloyl chloride In dichloromethane at 25 - 40℃; for 2h; | 95% |
| Stage #1: 3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one; 1-hexadecylcarboxylic acid With dmap; triethylamine In dichloromethane at 25 - 30℃; for 0.25h; Stage #2: With pivaloyl chloride In dichloromethane at 25 - 40℃; for 2h; | 95% |
-
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

-
-
84163-13-3
6-fluoro-3-(piperidin-4-yl)benzo[d]isoxazole hydrochloride

-
-
144598-75-4
paliperidone

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In methanol for 24h; Reflux; | 94% |
| With N-ethyl-N,N-diisopropylamine In methanol Product distribution / selectivity; Reflux; | 93% |
| With N-ethyl-N,N-diisopropylamine In methanol at 60℃; Reagent/catalyst; Inert atmosphere; | 90% |
-
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

-
-
124-63-0
methanesulfonyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 0.5h; Cooling with ice; | 93% |
-
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

-
-
144598-75-4
paliperidone

| Conditions | Yield |
|---|---|
| With diisopropylamine In methanol for 12h; Time; Inert atmosphere; Reflux; | 88% |
-
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

| Conditions | Yield |
|---|---|
| With 18-crown-6 ether; potassium carbonate In tetrahydrofuran Reflux; | 82% |
-
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

| Conditions | Yield |
|---|---|
| With diethylamino-sulfur trifluoride In dichloromethane at 20℃; for 3h; Cooling with ice; | 74% |
-
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

-
-
136434-34-9
duloxetine hydrochloride

| Conditions | Yield |
|---|---|
| With potassium carbonate; sodium iodide In N,N-dimethyl-formamide at 65℃; Inert atmosphere; | 72% |
-
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

-
-
135634-18-3
(2,4-difluorophenyl)-piperidin-4-yl-methanone oxime hydrochloride

-
-
1141761-80-9
3-(2-{4-((2,4-difluorophenyl)hydroxyiminomethyl)piperidin-1-yl}ethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-pyrido[1,2-a]pyrimidin-4-one

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In acetonitrile for 16h; Reflux; | 70% |
-
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

-
-
84163-77-9
6-fluoro-3-(4-piperidinyl)benzo[d]isoxazole

-
-
144598-75-4
paliperidone

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate; potassium iodide In water; acetonitrile at 65℃; for 16h; Reagent/catalyst; Solvent; Temperature; | 60% |
| With N-ethyl-N,N-diisopropylamine In methanol at 50 - 70℃; | 55% |
| With sodium carbonate In ethanol at 58 - 62℃; for 24h; Product distribution / selectivity; |

-
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In acetonitrile at 85℃; | 58% |
-
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In acetonitrile at 85℃; | 56% |
-
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In acetonitrile for 12h; Reflux; | 49% |
-
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

| Conditions | Yield |
|---|---|
| Stage #1: C27H31F2N5O4 In ethanol at 20 - 30℃; Acidic conditions; Stage #2: 3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one With N-ethyl-N,N-diisopropylamine In methanol Reflux; | 49% |
-
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In acetonitrile at 80℃; | 46.6% |
-
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

-
-
849727-63-5
(±)-3-(2-chloroethyl)-6,7,8,9-tetrahydro-9-hydroxy-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one monohydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water; butanone pH=2; | 46% |
-
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

| Conditions | Yield |
|---|---|
| Stage #1: C28H32F2N4O4 In ethanol at 20 - 30℃; Acidic conditions; Stage #2: 3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one With N-ethyl-N,N-diisopropylamine In methanol Reflux; | 42% |
-
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In acetonitrile Reflux; | 41% |
-
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

| Conditions | Yield |
|---|---|
| Stage #1: C28H32F2N4O4 In ethanol at 20 - 30℃; Acidic conditions; Stage #2: 3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one With N-ethyl-N,N-diisopropylamine In methanol Reflux; | 41% |
-
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In methanol Reflux; | 36% |
-
-
130049-82-0
3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In methanol Reflux; | 36% |
3-(2-Chloroethyl)-6,7,8,9-tetrahydro-9-hydroxy-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one Chemical Properties
Empirical Formula: C11H15ClN2O2
Molecular Weight: 242.702g/mol
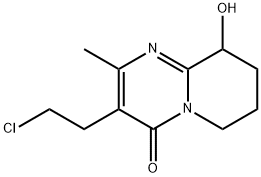
Structure of 4H-Pyrido[1,2-a]pyrimidin-4-one,3-(2-chloroethyl)-6,7,8,9-tetrahydro-9-hydroxy-2-methyl- (CAS NO.130049-82-0):
Index of Refraction: 1.634
Molar Refractivity: 61.26 cm3
Molar Volume: 171.1 cm3
Polarizability: 24.28×10-24cm3
Surface Tension: 50.3 dyne/cm
Density: 1.41 g/cm3
Flash Point: 189.2 °C
Enthalpy of Vaporization: 73.84 kJ/mol
Boiling Point: 389.3 °C at 760 mmHg
Vapour Pressure: 1.14E-07 mmHg at 25°C
Product Categories: Heterocyclic Compounds;Bases & Related Reagents;Heterocycles;Nucleotides
Systematic Name: 3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one
SMILES: O=C2C(/CCCl)=C(/C)\N=C1\C(O)CCCN12
InChI: InChI=1/C11H15ClN2O2/c1-7-8(4-5-12)11(16)14-6-2-3-9(15)10(14)13-7/h9,15H,2-6H2,1H3
InChIKey: JKVUGXRJSYRXFN-UHFFFAOYAK
3-(2-Chloroethyl)-6,7,8,9-tetrahydro-9-hydroxy-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one Specification
4H-Pyrido[1,2-a]pyrimidin-4-one,3-(2-chloroethyl)-6,7,8,9-tetrahydro-9-hydroxy-2-methyl- , its cas register number is 130049-82-0. It also can be called 3-(2-Chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one . 4H-Pyrido[1,2-a]pyrimidin-4-one,3-(2-chloroethyl)-6,7,8,9-tetrahydro-9-hydroxy-2-methyl- (CAS NO.130049-82-0) is a pale-yellow solid.
Related Products
- 3-(2-Chloroethyl)-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one
- 3-(2-Chloroethyl)-6,7,8,9-tetrahydro-9-hydroxy-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one
- 13005-11-3
- 1300-51-2
- 13005-29-3
- 13005-35-1
- 13005-36-2
- 130054-54-5
- 130064-21-0
- 130066-57-8
- 1300-71-6
- 1300-72-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View