-
Name
3-(3-Hydroxyphenyl)-DL-alanine
- EINECS 212-270-9
- CAS No. 775-06-4
- Article Data42
- CAS DataBase
- Density 1.333 g/cm3
- Solubility
- Melting Point 280-285 °C (dec.)(lit.)
- Formula C9H11NO3
- Boiling Point 387.2 °C at 760 mmHg
- Molecular Weight 181.191
- Flash Point 188 °C
- Transport Information
- Appearance white crystals powder
- Safety 26-36-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms DL-Metatyrosine;DL-m-Hydroxyphenylalanine;DL-m-Tyrosine;DL-a-m-Tyrosine;Metatyrosine;NSC 89304;m-DL-Tyrosine;m-Hydroxyphenylalanine;m-Tyrosine;DL-Phenylalanine,3-hydroxy-;m-Tyrosine, DL- (8CI);2-Amino-3-(3-hydroxyphenyl)propanoic acid;DL-3-(m-Hydroxyphenyl)alanine;DL-3-Hydroxyphenylalanine;
- PSA 83.55000
- LogP 1.04690
Synthetic route

| Conditions | Yield |
|---|---|
| With iron sulfide; ammonium carbonate In water at 100℃; for 336h; | 45% |
-
-
41888-66-8
4-(3-acetoxy-benzylidene)-2-methyl-4H-oxazol-5-one

-
-
775-06-4
D/L-3-hydroxy-phenylalanine

| Conditions | Yield |
|---|---|
| With phosphorus; hydrogen iodide; acetic acid | |
| With sodium amalgam |
-
-
28101-74-8
3-aminophenylalanine

-
-
775-06-4
D/L-3-hydroxy-phenylalanine

| Conditions | Yield |
|---|---|
| With potassium nitrite Diazotization; |
-
-
50713-75-2
N-benzoyl-3-hydroxy-phenylalanine

-
-
775-06-4
D/L-3-hydroxy-phenylalanine

| Conditions | Yield |
|---|---|
| With hydrogenchloride |

| Conditions | Yield |
|---|---|
| With phosphorus; hydrogen iodide |
-
-
150-30-1
Phenylalanine

-
A
-
775-06-4
D/L-3-hydroxy-phenylalanine

-
B
-
709-16-0, 2370-61-8, 7423-92-9, 24008-77-3
2-hydroxy-phenylalanine

| Conditions | Yield |
|---|---|
| With 6,7-dimethyl-5,6,7,8-tetrahydropteridine; oxygen at 37℃; Kinetics; in citrate buffer(pH 6.0), also with MPH4 and in the pH range of 3 to 8,effect of various substances; | |
| With hypoxanthine; xanthine at 37℃; for 0.5h; Mechanism; in citrate buffer(pH 5.5), also in the pH range of 4.5 to 6.5, effect ofsuperoxide dismutase, catalase and various substances; |

-
-
150-30-1
Phenylalanine

-
A
-
775-06-4
D/L-3-hydroxy-phenylalanine

-
B
-
709-16-0, 2370-61-8, 7423-92-9, 24008-77-3
2-hydroxy-phenylalanine

-
D
-
56-40-6
glycine

-
E
-
617-45-8
aspartic Acid

| Conditions | Yield |
|---|---|
| In water for 0.333333h; Product distribution; hydroxylation of the aromatic ring was investigated by the action hydroxyl radicals generated by decomposition of water molecules by the high energy plasma, and other systems.; |


-
-
150-30-1
Phenylalanine

-
A
-
775-06-4
D/L-3-hydroxy-phenylalanine

-
B
-
709-16-0, 2370-61-8, 7423-92-9, 24008-77-3
2-hydroxy-phenylalanine

| Conditions | Yield |
|---|---|
| γ-Strahlen.Irradiation; |


| Conditions | Yield |
|---|---|
| With barium dihydroxide |

-
-
775-06-4
D/L-3-hydroxy-phenylalanine

| Conditions | Yield |
|---|---|
| With barium dihydroxide |
-
-
150034-36-9
α-benzoylamino-3-hydroxy-cinnamic acid

-
-
775-06-4
D/L-3-hydroxy-phenylalanine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: water; sodium amalgam 2: hydrochloric acid View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium acetate 2: aqueous HI; red phosphorus; acetic acid View Scheme | |
| Multi-step reaction with 2 steps 1: acetic acid anhydride; sodium acetate / 135 - 140 °C 2: hydriodic acid; red phosphorus View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: hydriodic acid; red phosphorus 2: potassium nitrite / Diazotization View Scheme |
-
-
150-30-1
Phenylalanine

-
A
-
775-06-4
D/L-3-hydroxy-phenylalanine

-
B
-
709-16-0, 2370-61-8, 7423-92-9, 24008-77-3
2-hydroxy-phenylalanine

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; copper(II) sulfate; aminoguanidine In phosphate buffer at 37℃; for 4h; pH=7.4; Reactivity; | |
| With dihydrogen peroxide; copper(II) sulfate; pyridoxamine In phosphate buffer at 37℃; for 4h; pH=7.4; Reactivity; | |
| With dihydrogen peroxide; copper(II) sulfate; olmesartan In phosphate buffer at 37℃; for 4h; pH=7.4; Reactivity; | |
| With dihydrogen peroxide; copper(II) sulfate; 1-(5-hydroxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)-6-methyl-1,3-dihydro-furo[3,4-c]pyridin-7-ol In phosphate buffer at 37℃; for 4h; pH=7.4; Reactivity; |
-
-
14755-02-3
3-hydroxy-trans-cinnamic acid

-
-
775-06-4
D/L-3-hydroxy-phenylalanine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: ammonium hydroxide / 2 h / 30 °C / pH 9.5 / Enzymatic reaction 2: Pseudomonas putida phenylalanine racemase / Enzymatic reaction View Scheme |
-
-
587-33-7, 775-06-4, 2180-37-2, 32140-49-1
m-tyrosine

-
-
775-06-4
D/L-3-hydroxy-phenylalanine

| Conditions | Yield |
|---|---|
| With Pseudomonas putida phenylalanine racemase Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| With recombinant FsqB flavoprotein from Aspergillus fumigatus containing covalently bound FAD cofactor; oxygen In aq. phosphate buffer Kinetics; Enzymatic reaction; | A 24 %Chromat. B 28 %Chromat. |
-
-
6720-12-3
rac-N-methyl-meta-tyrosine

-
A
-
775-06-4
D/L-3-hydroxy-phenylalanine

-
C
-
76824-99-2
6-hydroxy-1,2,3,4-tetrahydro-3-isoquinoline carboxylic acid

| Conditions | Yield |
|---|---|
| With recombinant FsqB flavoprotein from Aspergillus fumigatus, D444A mutant, containing covalently bound FAD cofactor; oxygen In aq. phosphate buffer Enzymatic reaction; | A 16 %Chromat. B 23 %Chromat. C 12 %Chromat. |

-
-
64-17-5
ethanol

-
-
775-06-4
D/L-3-hydroxy-phenylalanine

-
-
35532-01-5
ethyl 2-amino-3-(3-hydroxyphenyl)propanoate hydrochloride

| Conditions | Yield |
|---|---|
| With thionyl chloride at -5℃; Reflux; | 99% |
| With thionyl chloride at 5 - 20℃; for 18h; Heating / reflux; | 95% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide Acylation; | 98% |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
775-06-4
D/L-3-hydroxy-phenylalanine

-
-
174732-96-8
Nα-Boc-D,L-m-tyrosine

| Conditions | Yield |
|---|---|
| With triethylamine In 1,4-dioxane; water at -2 - 0℃; | 97% |
| With triethylamine In 1,4-dioxane; water at 0℃; | 97% |
| With triethylamine In 1,4-dioxane; water at 20℃; for 4h; | 93.4% |
| With triethylamine In 1,4-dioxane; water for 16h; | 90% |

| Conditions | Yield |
|---|---|
| With thionyl chloride at 0 - 75℃; for 21h; | 96% |
| With hydrogenchloride at 20℃; for 2h; |

| Conditions | Yield |
|---|---|
| With sodium carbonate In methanol at 65℃; diastereoselective reaction; | 93% |

-
-
50-00-0
formaldehyd

-
-
775-06-4
D/L-3-hydroxy-phenylalanine

-
-
76824-99-2
6-hydroxy-1,2,3,4-tetrahydro-3-isoquinoline carboxylic acid

| Conditions | Yield |
|---|---|
| With water at 20℃; Picted-Spengler reaction; | 89% |
| With hydrogenchloride In water at 100℃; | 48% |
-
-
775-06-4
D/L-3-hydroxy-phenylalanine

-
A
-
587-33-7, 775-06-4, 2180-37-2, 32140-49-1
m-tyrosine

-
B
-
32140-49-1
m-hydroxy-D-phenylalanine

| Conditions | Yield |
|---|---|
| With α-chymotrypsin | A 89% B 85% |
| With α-chymotrypsin 1.) enzymatic hydrolysis by α-chymotrypsin; Yield given. Multistep reaction; | |
| With (S)-2-hydroxy-2'-(3-(N-phenylcarbamoylamino)benzyl)-1,1'-binaphthyl-3-carboxaldehyde In dimethyl sulfoxide Resolution of racemate; stereoselective reaction; | A n/a B n/a |
| With (R)-2-hydroxy-2'-(3-phenylurylbenzyl)-1,1'-binaphthyl-3-carboxaldehyde In dimethyl sulfoxide Resolution of racemate; stereoselective reaction; | A n/a B n/a |
-
-
775-06-4
D/L-3-hydroxy-phenylalanine

-
-
123254-09-1
2-amino-3-(2-bromo-5-hydroxyphenyl)propanoic acid

| Conditions | Yield |
|---|---|
| With bromine In acetic acid at 20℃; | 70% |
| With bromine; acetic acid | |
| With bromine In acetic acid | |
| With bromine; acetic acid In water at 20℃; for 2h; Inert atmosphere; | |
| With bromine; acetic acid for 16h; |
-
-
50-00-0
formaldehyd

-
-
775-06-4
D/L-3-hydroxy-phenylalanine

-
B
-
76824-99-2
6-hydroxy-1,2,3,4-tetrahydro-3-isoquinoline carboxylic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 0.75h; Heating; | A n/a B 70% |

-
-
775-06-4
D/L-3-hydroxy-phenylalanine

| Conditions | Yield |
|---|---|
| With sodiumsulfide nonahydrate; water; iodine; acetic acid In dimethyl sulfoxide at 100℃; for 8h; | 70% |
-
-
775-06-4
D/L-3-hydroxy-phenylalanine

-
-
100-51-6
benzyl alcohol

-
-
127153-04-2
(+/-)-3-hydroxyphenylalanine phenylmethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: benzyl alcohol With thionyl chloride at -5℃; Stage #2: D/L-3-hydroxy-phenylalanine at -5 - 120℃; Stage #3: With sodium hydrogencarbonate In ethyl acetate | 59% |
| With toluene-4-sulfonic acid In toluene for 4h; Heating; | 8.72 g |

| Conditions | Yield |
|---|---|
| With triethylamine In 1,2-dimethoxyethane; water at 20℃; Condensation; | 45.5% |

| Conditions | Yield |
|---|---|
| With perchloric acid at 0 - 25℃; for 10h; | 33.21% |
-
-
64-18-6
formic acid

-
-
775-06-4
D/L-3-hydroxy-phenylalanine

-
-
72683-77-3, 72683-78-4
N-formyl-3-hydroxy-DL-phenylalanine

| Conditions | Yield |
|---|---|
| With acetic anhydride |

| Conditions | Yield |
|---|---|
| Irradiation.UV-Licht; |
-
-
775-06-4
D/L-3-hydroxy-phenylalanine

-
-
91054-28-3
5-hydroxy-2,4-diiodo-phenylalanine

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; water; iodine; potassium iodide |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; diethyl ether |

| Conditions | Yield |
|---|---|
| With NH4OH-NH4Cl buffer; pyridoxal 5'-phosphate at 30℃; for 0.5h; relative rate of CO2 evolution by aromatic L-amino acid decarboxylase from Micrococcus percitreus; |

| Conditions | Yield |
|---|---|
| With 0,2,4-dichlorophenyl-0-methylisopropylamidothiophosphate; hydroxide; dicyclohexyl-carbodiimide Multistep reaction; |


| Conditions | Yield |
|---|---|
| With 0,2,4-dichlorophenyl-0-methylisopropylamidothiophosphate; hydroxide; dicyclohexyl-carbodiimide Multistep reaction; |

-
-
775-06-4
D/L-3-hydroxy-phenylalanine

-
A
-
103753-98-6
3-hydroxy-2,4-dinitro-phenylalanine

-
B
-
103752-68-7
5-hydroxy-2,4-dinitro-phenylalanine

| Conditions | Yield |
|---|---|
| With nitronium tetrafluoborate In acetonitrile 1.) 0 deg C, 2 h, 2.) RT, 5.5 h; | |
| With nitronium tetrafluoborate In acetonitrile 1.) 0 deg C, 2h, 2.) RT, 5.5 h; |

3-(3-Hydroxyphenyl)-DL-alanine Chemical Properties
Structure of DL-m-Tyrosine (CAS NO.775-06-4):
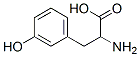
IUPAC Name: 2-Amino-3-(3-hydroxyphenyl)propanoic acid
Empirical Formula: C9H11NO3
Molecular Weight: 181.1885 g/mol
Index of Refraction: 1.614
Density: 1.333 g/cm3
Flash Point: 188 °C
Melting point: 280-285 °C (dec.)(lit.)
Appearance: White crystals powder
Boiling Point: 387.2 °C at 760 mmHg
Vapour Pressure: 1.09E-06 mmHg at 25 °C
Product Category: Tyrosine [Tyr, Y];Unusual Amino Acids;Amino Acids & Derivatives
3-(3-Hydroxyphenyl)-DL-alanine Uses
DL-m-Tyrosine (CAS NO.775-06-4) has been used for the precursor in the formation of catecholamines.
3-(3-Hydroxyphenyl)-DL-alanine Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intravenous | 320mg/kg (320mg/kg) | U.S. Army Armament Research & Development Command, Chemical Systems Laboratory, NIOSH Exchange Chemicals. Vol. NX#02549, |
3-(3-Hydroxyphenyl)-DL-alanine Safety Profile
Hazard Codes:  Xi
Xi
Risk Statements: 36/37/38
R36/37/38: Irritating to eyes, respiratory system and skin.
Safety Statements: 26-36-37/39
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36: Wear suitable protective clothing.
S37/39: Wear suitable gloves and eye/face protection.
3-(3-Hydroxyphenyl)-DL-alanine Specification
DL-m-Tyrosine ,its cas register number is 775-06-4. It also can be called (+/-)-2-Amino-3-(3-hydroxyphenyl)propionic acid ; 3-Hydroxyphenylalanine ; Meta-Tyrosine ; 3-Tyrosine ; and 3-(3-Hydroxyphenyl)-DL-alanine . It is hazardous,so the first aid measures and others should be known. Such as: When on the skin: first,should flush skin with plenty of water immediately for at least 15 minutes while removing contaminated clothing. Secondly,Get medical aid. Or in the eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Then get medical aid soon. While ,it's Inhaled: Remove from exposure and move to fresh air immediately.Give artificial respiration while not breathing. When breathing is difficult, give oxygen. And as soon as to get medical aid. Then you have the ingesting of the product : Wash mouth out with water,and get medical aid immediately.Notes to physician: Treat supportively and symptomatically.
In addition, DL-m-Tyrosine (CAS NO.775-06-4) could be stable under normal temperatures and pressures. It is not compatible with strong oxidizing agents, and you must not take it with incompatible materials. And also prevent it to broken down into hazardous decomposition products: nitrogen oxides, carbon monoxide, carbon dioxide.
Related Products
- 3-(3-Hydroxyphenyl)-DL-alanine
- 77-51-0
- 77510-50-0
- 775-12-2
- 7751-38-4
- 77514-31-9
- 775-15-5
- 775-16-6
- 77-52-1
- 77521-29-0
- 77522-86-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View