-
Name
3-(Methylamino)toluene
- EINECS 211-795-0
- CAS No. 696-44-6
- Article Data68
- CAS DataBase
- Density 0.968 g/cm3
- Solubility insoluble
- Melting Point -10.08°C (estimate)
- Formula C8H11N
- Boiling Point 204.7 °C at 760 mmHg
- Molecular Weight 121.182
- Flash Point 77.3 °C
- Transport Information UN 2810
- Appearance clear yellow liquid
- Safety 28-36/37-45-61-28A
- Risk Codes 23/24/25-33-52/53
-
Molecular Structure
-
Hazard Symbols
 T
T
- Synonyms m-Toluidine,N-methyl- (6CI,7CI,8CI);N,3-Dimethylaniline;N,3-Dimethylbenzenamine;N-(3-Methylphenyl)methylamine;N-Methyl(3-methylphenyl)amine;N-Methyl-N-(3-methylphenyl)amine;N-Methyl-N-(m-tolyl)amine;N-Methyl-m-toluidine;NSC 9396;m,N-Dimethylaniline;
- PSA 12.03000
- LogP 2.10970
Synthetic route

| Conditions | Yield |
|---|---|
| With C31H30ClIrN3O(1+)*F6P(1-); potassium tert-butylate at 130℃; for 12h; | 98% |
| With C19H37IrN4(2+)*2I(1-); potassium carbonate at 120℃; for 17h; Inert atmosphere; Schlenk technique; Sealed tube; | 90% |
| With triphenylphosphine; 2,3-dicyano-5,6-dichloro-p-benzoquinone In dichloromethane at 20℃; | |
| With hydrogen at 55℃; under 750.075 Torr; for 36h; Irradiation; Green chemistry; | |
| With C19H24ClN2Ru; sodium hydroxide at 60℃; for 22h; | 99 %Chromat. |
-
-
579-12-4
3-methyl-N-methylacetanilide

-
A
-
15258-50-1
N-ethyl-N-methyl-3-methylaniline

-
B
-
696-44-6
N-methyl-m-toluidine

| Conditions | Yield |
|---|---|
| With triethyl borane; Triethoxysilane; sodium hydroxide In hexane at 80℃; for 6h; Reagent/catalyst; Solvent; Inert atmosphere; Sealed tube; | A 2% B 95% |
| Stage #1: 3-methyl-N-methylacetanilide With triethyl borane; 4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane; potassium hydroxide In tetrahydrofuran at 100℃; for 24h; Inert atmosphere; Schlenk technique; Sealed tube; Stage #2: With water; sodium hydroxide In tetrahydrofuran at 25℃; for 1h; Inert atmosphere; Schlenk technique; Sealed tube; | A 78% B 11% |
| With sodium triethylborohydride In tetrahydrofuran at 80℃; for 6h; Inert atmosphere; Schlenk technique; High pressure; Sealed tube; | A n/a B 72% |

| Conditions | Yield |
|---|---|
| Stage #1: formaldehyd; 1-amino-3-methylbenzene With sodium methylate In methanol at 65℃; for 1h; Stage #2: With sodium tetrahydroborate at 65℃; for 1.5h; | 93% |
| With sodium tetrahydroborate; sodium methylate In methanol 1) room temp., 5 h, 2) reflux; | 89% |
| With methanol; sodium tetrahydroborate; sodium at 60℃; for 2h; | 82% |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate at 130℃; for 24h; Sealed tube; | 93% |
| With [(N,N′-bis(diisopropylphosphino)-2,6-diaminopyridine)Mn(CO)3][Br]; potassium hydroxide at 110℃; for 16h; Molecular sieve; Sealed tube; | 80% |
| With [Ru(2-(6-methoxypyridin-2-yl)-1,10-phenanthroline)(MeCN)2Cl]Cl; sodium methylate at 110℃; for 12h; Green chemistry; | 87 %Chromat. |
| With platinum on carbon; potassium tert-butylate; hydrogen at 150℃; under 1500.15 Torr; for 24h; Autoclave; | 93 %Chromat. |
-
-
68-11-1
mercaptoacetic acid

-
-
211676-01-6
N-methyl-N-nosyl-m-toluidine

-
A
-
3406-75-5
2-(4-nitrophenylthio)acetic acid

-
B
-
696-44-6
N-methyl-m-toluidine

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In acetonitrile at 20℃; | A n/a B 92% |


| Conditions | Yield |
|---|---|
| With copper(l) iodide; 6,7-dihydro-5H-quinolin-8-one oxime; potassium hydroxide In water at 25℃; for 24h; Inert atmosphere; | 90% |
-
-
67-56-1
methanol

-
-
108-44-1
1-amino-3-methylbenzene

-
A
-
121-72-2
N,N-dimethyl-m-toluidine

-
B
-
696-44-6
N-methyl-m-toluidine

| Conditions | Yield |
|---|---|
| With copper(I) oxide; hydrogenchloride; titanium(IV) oxide In water for 6h; UV-irradiation; | A 83% B 15% |
| With aluminum oxide at 350 - 400℃; |


-
-
696-44-6
N-methyl-m-toluidine

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In dichloromethane at 25℃; | 83% |
| With trifluoroacetic acid In dichloromethane at 40℃; for 48h; Inert atmosphere; Schlenk technique; | |
| With hydrogenchloride In water at 120℃; |

-
A
-
1912-16-9
2-methyl-4,6-diphenyl-pyridine

-
B
-
623-08-5
N-methyl-p-toluidine

-
C
-
696-44-6
N-methyl-m-toluidine

-
D
-
611-21-2
N,2-dimethylaniline

| Conditions | Yield |
|---|---|
| In trifluoroacetic acid for 17h; Ambient temperature; Irradiation; | A 81% B n/a C n/a D n/a |

-
-
616-38-6
carbonic acid dimethyl ester

-
-
108-44-1
1-amino-3-methylbenzene

-
A
-
121-72-2
N,N-dimethyl-m-toluidine

-
B
-
696-44-6
N-methyl-m-toluidine

| Conditions | Yield |
|---|---|
| With 0.72KNaX-BS zeolite at 150℃; for 1h; Temperature; | A 77% B 22% |
| With NaY-BS zeolite at 150℃; for 1h; | A 32% B 43% |


| Conditions | Yield |
|---|---|
| Stage #1: methanol; 3-methylbenzoyl azide at 70℃; for 3h; Inert atmosphere; Stage #2: With C54H43ClN3P2Ru(1+)*F6P(1-); potassium carbonate at 140℃; Inert atmosphere; | 77% |

| Conditions | Yield |
|---|---|
| With sodium triethylborohydride In tetrahydrofuran at 80℃; for 12h; Inert atmosphere; Schlenk technique; Glovebox; Sealed tube; | 72% |
| With hydrogenchloride In water; ethylene glycol at 20℃; for 3h; Inert atmosphere; |
-
-
616-38-6
carbonic acid dimethyl ester

-
-
108-44-1
1-amino-3-methylbenzene

-
-
696-44-6
N-methyl-m-toluidine

| Conditions | Yield |
|---|---|
| With 0.94 HY-BS zeolite at 150℃; for 1h; | 67% |
| With binder-free FeHY-mmm zeolite In neat (no solvent) Sealed tube; |

-
B
-
696-44-6
N-methyl-m-toluidine

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide In tetrahydrofuran; hexane at -78℃; for 3h; [1,2]-Wittig Rearrangement; | A 62% B 9% |
-
-
5827-78-1
N-methyl-N-phenylpropanamide

-
A
-
13395-54-5
N-methyl-N-n-propylaniline

-
B
-
696-44-6
N-methyl-m-toluidine

| Conditions | Yield |
|---|---|
| Stage #1: N-methyl-N-phenylpropanamide With triethyl borane; 4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane; potassium hydroxide In tetrahydrofuran at 100℃; for 24h; Inert atmosphere; Schlenk technique; Sealed tube; Stage #2: With water; sodium hydroxide In tetrahydrofuran at 25℃; for 1h; Inert atmosphere; Schlenk technique; Sealed tube; | A 61% B 11% |

| Conditions | Yield |
|---|---|
| With copper In water at 100℃; | 52% |
-
-
87117-15-5
1-methoxy-2,6-dimethylpyridinium methyl sulfate

-
A
-
108-48-5
2,6-dimethylpyridine

-
B
-
696-44-6
N-methyl-m-toluidine

| Conditions | Yield |
|---|---|
| With Dimethylammonium sulphite In water at 150℃; for 24h; | A 20% B 42% |

| Conditions | Yield |
|---|---|
| man befreit das Produkt durch Ausfaellen seiner aether.Loesung mit Schwefelsaeure vom Toluidin, dampft die aether.Loesung ein, erhitzt den Rueckstand mit Eissigsaeureanhydrid und verseift das entstandene N-Methyl-; | |
| Stage #1: 1-amino-3-methylbenzene With n-butyllithium In tetrahydrofuran; hexane at -40℃; for 0.25h; Inert atmosphere; Stage #2: methyl iodide In tetrahydrofuran; hexane at -60 - 20℃; for 8h; Inert atmosphere; |
-
-
108-44-1
1-amino-3-methylbenzene

-
-
74-88-4
methyl iodide

-
A
-
121-72-2
N,N-dimethyl-m-toluidine

-
B
-
696-44-6
N-methyl-m-toluidine


| Conditions | Yield |
|---|---|
| With sulfuric acid unter Entfernen des entstehenden Methanols auf 180grad und Erhitzen des danach isolierten Reaktionsprodukts mit wss.Salzsaeure; |
-
-
39970-42-8
N-methyl-N-(3-methylphenyl)carboxamide

-
-
696-44-6
N-methyl-m-toluidine

| Conditions | Yield |
|---|---|
| With water Heating; Yield given; | |
| With hydrogenchloride In water at 100℃; for 2.5h; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 30℃; Rate constant; Mechanism; also aq. DMSO; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water; dimethyl sulfoxide at 90℃; Rate constant; Mechanism; |
-
-
79431-18-8
(Dimethoxy-phenyl-methyl)-methyl-m-tolyl-amine

-
A
-
38092-03-4, 102651-95-6
α,α-dimethoxybenzyl cation

-
B
-
696-44-6
N-methyl-m-toluidine

| Conditions | Yield |
|---|---|
| With water In 1,4-dioxane at 25℃; Rate constant; |

| Conditions | Yield |
|---|---|
| With dimethylsulfide borane complex 1) THF, r.t., 2 h, 2) THF, reflux, 3 h; Multistep reaction; |

| Conditions | Yield |
|---|---|
| With dimethylsulfide borane complex 1.) THF, RT, 2h, 2.) THF, reflux, 3 h; Multistep reaction; | |
| Multi-step reaction with 3 steps 1: benzene / Heating 2: anhydrous potassium carbonate, sodium hydroxide, tetrabutylammonium hydrogensulphate / benzene / 1 h / 5 - 10 °C / Heating 3: water / Heating View Scheme | |
| Multi-step reaction with 3 steps 1.1: tert-butyl alcohol / 24 h / 40 °C / Inert atmosphere; Schlenk technique 2.1: sodium hydride / N,N-dimethyl-formamide; mineral oil / 0.33 h / 0 °C / Inert atmosphere; Schlenk technique 2.2: 20 °C / Inert atmosphere; Schlenk technique 3.1: trifluoroacetic acid / dichloromethane / 48 h / 40 °C / Inert atmosphere; Schlenk technique View Scheme |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; N-benzyl-N,N,N-triethylammonium chloride 1.) benzene, water, 30 min, 2.) 2 h.; Multistep reaction; |
-
-
67-56-1
methanol

-
-
108-44-1
1-amino-3-methylbenzene

-
A
-
121-72-2
N,N-dimethyl-m-toluidine

-
B
-
696-44-6
N-methyl-m-toluidine

| Conditions | Yield |
|---|---|
| at 350 - 400℃; |


-
A
-
14088-79-0
N-(2-aminoethyl)-3-methylaniline

-
B
-
696-44-6
N-methyl-m-toluidine

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride; diethyl ether |
-
-
696-44-6
N-methyl-m-toluidine

-
-
17485-25-5
3-methyl-N-nitroso-N-methylaniline

| Conditions | Yield |
|---|---|
| With sodium azide In water; acetonitrile at 20℃; | 100% |
| With nitromethane; oxygen; copper(II) bis(trifluoromethanesulfonate); 1,8-diazabicyclo[5.4.0]undec-7-ene at 90℃; for 20h; Sealed tube; | 63% |
| With hydrogenchloride; sodium nitrite at 0 - 5℃; | 2.5% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; Inert atmosphere; | 100% |
| With triethylamine In dichloromethane at 0 - 20℃; | 99% |
-
-
696-44-6
N-methyl-m-toluidine

-
-
51154-06-4
(S)-2,5-dihydropyrrole-1,2-dicarboxylic acid 1-tert-butyl ester

| Conditions | Yield |
|---|---|
| With pyridine; 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide In ethyl acetate at 0 - 20℃; for 4h; | 100% |

| Conditions | Yield |
|---|---|
| With dichloro(dimethylglyoxime)(dimethylglyoximato)cobalt(III); (4,4'-di-tert-butyl-2,2'-dipyridyl)-bis-(2-phenylpyridine(-1H))-iridium(III) hexafluorophosphate; triethylamine In acetonitrile at -78℃; for 24h; Reagent/catalyst; Sealed tube; Inert atmosphere; Irradiation; | 100% |
-
-
696-44-6
N-methyl-m-toluidine

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
-
39970-42-8
N-methyl-N-(3-methylphenyl)carboxamide

| Conditions | Yield |
|---|---|
| With zinc(II) phthalocyanine; carbon dioxide; phenylsilane at 25℃; under 3750.38 Torr; for 6h; Autoclave; chemoselective reaction; | 99% |
-
-
696-44-6
N-methyl-m-toluidine

-
-
3433-80-5
1-Bromo-2-bromomethyl-benzene

-
-
436845-45-3
N-(2-bromobenzyl)-N-methyl-m-tolylamine

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile for 4h; Heating; | 98% |
-
-
31555-60-9
5-bromothiophene-2-carbonyl chloride

-
-
696-44-6
N-methyl-m-toluidine

-
-
1252464-84-8
5-bromo-N-methyl-N-(3-methylphenyl)thiophene-2-carboxamide

| Conditions | Yield |
|---|---|
| With triethylamine at 0℃; for 3h; | 97% |
| With triethylamine In dichloromethane at 0℃; for 0.5h; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With aluminum oxide; aluminium trichloride for 0.0666667h; microwave irradiation; | 96% |

| Conditions | Yield |
|---|---|
| With diphenylsilane; caesium carbonate In acetonitrile at 80℃; under 750.075 Torr; for 72h; Schlenk technique; Inert atmosphere; | 95% |
| With phenylsilane; triphenylphosphine In tetrahydrofuran at 120℃; under 3750.38 Torr; for 24h; Autoclave; Green chemistry; | 93% |
| With dimanganese decacarbonyl; phenylsilane; C29H33N2P In acetonitrile at 100℃; under 750.075 Torr; for 15h; | 88% |
-
-
1444336-77-9
3-(benzyloxy)-6-bromo-2,4,5-trimethylpyridine

-
-
696-44-6
N-methyl-m-toluidine

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl; sodium t-butanolate In toluene for 3h; Reflux; | 95% |
-
-
124-38-9
carbon dioxide

-
-
696-44-6
N-methyl-m-toluidine

-
-
39970-42-8
N-methyl-N-(3-methylphenyl)carboxamide

| Conditions | Yield |
|---|---|
| With 1,10-Phenanthroline; phenylsilane; zinc diacetate In acetonitrile at 25℃; under 3750.38 Torr; for 4h; Autoclave; | 95% |
| With 2K(1+)*VOF4(2-)=K2{VOF4}; HSiPh3 In acetonitrile at 20℃; under 750.075 Torr; for 10h; Sealed tube; Autoclave; | 95% |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In acetonitrile at 30℃; under 760.051 Torr; for 72h; Schlenk technique; | 93% |

| Conditions | Yield |
|---|---|
| With potassium phosphate; 18-crown-6 ether; phenylsilane In tetrahydrofuran at 80℃; for 12h; Glovebox; Molecular sieve; Schlenk technique; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: N-methyl-m-toluidine With N,N'-bis(2,6-dicyclopentyl-4-methoxyphenyl)formamidine; tetrabenzyl titanium at 20℃; for 0.333333h; Glovebox; Inert atmosphere; Stage #2: oct-1-ene at 155℃; for 0.116667h; Sealed tube; | 94% |
| With bis(indenyl)dimethyl-titanium In toluene at 105℃; for 24h; Inert atmosphere; regioselective reaction; | 84% |
-
-
696-44-6
N-methyl-m-toluidine

| Conditions | Yield |
|---|---|
| With water-d2; silver trifluoromethanesulfonate In chloroform-d1 at 90℃; for 18h; regioselective reaction; | 94% |

| Conditions | Yield |
|---|---|
| With C52H52NO5P; palladium(II) iodide In tetrahydrofuran at 20℃; under 38002.6 Torr; for 72h; Autoclave; enantioselective reaction; | 94% |


| Conditions | Yield |
|---|---|
| Stage #1: cyanoacetic acid With oxalyl dichloride; N,N-dimethyl-formamide In dichloromethane at 20℃; for 3h; Stage #2: N-methyl-m-toluidine With triethylamine In dichloromethane at 0 - 20℃; | 93% |
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0℃; for 1h; Inert atmosphere; Schlenk technique; | 81% |
| Stage #1: cyanoacetic acid With oxalyl dichloride In dichloromethane; N,N-dimethyl-formamide at 0 - 20℃; for 3h; Stage #2: N-methyl-m-toluidine With triethylamine In dichloromethane; N,N-dimethyl-formamide at 0℃; |

| Conditions | Yield |
|---|---|
| With pentakis(dimethylamido)tantalum In hexane at 140℃; for 96h; Inert atmosphere; Sealed tube; regioselective reaction; | 93% |

| Conditions | Yield |
|---|---|
| With C38H38N4O2Rh(1+)*F6P(1-) In acetonitrile at 20℃; for 7h; Mannich Aminomethylation; enantioselective reaction; | 93% |

-
-
35349-68-9
9-hydroxycalabaxanthone

-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
696-44-6
N-methyl-m-toluidine

| Conditions | Yield |
|---|---|
| Stage #1: bis(trichloromethyl) carbonate; N-methyl-m-toluidine With triethylamine In dichloromethane at 20℃; for 4h; Cooling with ice; Stage #2: 9-hydroxycalabaxanthone With dmap In dichloromethane at 20℃; Cooling with ice; | 92% |

-
-
696-44-6
N-methyl-m-toluidine

| Conditions | Yield |
|---|---|
| With bis(1,5-cyclooctadiene)nickel (0); (R)-segphos In tert-Amyl alcohol at 60℃; for 48h; Glovebox; enantioselective reaction; | 91% |

| Conditions | Yield |
|---|---|
| With potassium phosphate; 18-crown-6 ether; phenylsilane In tetrahydrofuran at 80℃; for 12h; Glovebox; Molecular sieve; Schlenk technique; | 90% |
-
-
884866-01-7
1-(4-methylbenzensulfonyl)-4-phenyl-1H-1,2,3-triazole

-
-
696-44-6
N-methyl-m-toluidine

| Conditions | Yield |
|---|---|
| In toluene at 120℃; for 12h; Inert atmosphere; | 89% |
-
-
667-27-6
Ethyl bromodifluoroacetate

-
-
696-44-6
N-methyl-m-toluidine

-
-
39970-42-8
N-methyl-N-(3-methylphenyl)carboxamide

| Conditions | Yield |
|---|---|
| With water In N,N-dimethyl-formamide at 110℃; for 6h; Schlenk technique; | 89% |
| With copper (I) acetate; caesium carbonate; XPhos In N,N-dimethyl-formamide at 110℃; for 12h; | 79% |
| With copper (I) acetate; caesium carbonate; XPhos In water; N,N-dimethyl-formamide at 110℃; for 12h; Schlenk technique; | 79% |
-
-
1955-39-1
5-phenyl-3H-furan-2-one

-
-
696-44-6
N-methyl-m-toluidine

-
-
1056199-48-4
N-methyl-N-(3-methylphenyl)-4-oxo-4-phenylbutanamide

| Conditions | Yield |
|---|---|
| for 3h; Heating; | 87% |
3-(Methylamino)toluene Chemical Properties
Molecule structure of 3-(Methylamino)toluene (CAS NO.696-44-6):
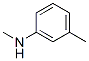
IUPAC Name: N,3-Dimethylaniline
Molecular Weight: 121.17964 g/mol
Molecular Formula: C8H11N
Density: 0.968 g/cm3
Boiling Point: 204.7 °C at 760 mmHg
Flash Point: 77.3 °C
Index of Refraction: 1.563
Molar Refractivity: 40.68 cm3
Molar Volume: 125.1 cm3
Surface Tension: 34.7 dyne/cm
Enthalpy of Vaporization: 44.1 kJ/mol
Vapour Pressure: 0.259 mmHg at 25 °C
Water Solubility: insoluble
XLogP3: 2.6
H-Bond Donor: 1
H-Bond Acceptor: 1
Rotatable Bond Count: 1
Exact Mass: 121.089149
MonoIsotopic Mass: 121.089149
Topological Polar Surface Area: 12
Heavy Atom Count: 9
Canonical SMILES: CC1=CC(=CC=C1)NC
InChI: InChI=1S/C8H11N/c1-7-4-3-5-8(6-7)9-2/h3-6,9H,1-2H3
InChIKey: FBGJJTQNZVNEQU-UHFFFAOYSA-N
EINECS: 211-795-0
3-(Methylamino)toluene Uses
3-(Methylamino)toluene (CAS NO.696-44-6) is used as dye intermediates.
3-(Methylamino)toluene Safety Profile
Hazard Codes:  T
T
Risk Statements: 23/24/25-33-52/53
R23/24/25:Toxic by inhalation, in contact with skin and if swallowed.
R33:Danger of cumulative effects.
R52/53:Harmful to aquatic organisms, may cause long-term adverse effects in the aquatic environment.
Safety Statements: 28-36/37-45-61-28A
S28:After contact with skin, wash immediately with plenty of soap-suds.
S36/37:Wear suitable protective clothing and gloves.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S61:Avoid release to the environment. Refer to special instructions / safety data sheets.
RIDADR: UN 2810 6.1/PG 3
WGK Germany: 3
HazardClass: 6.1(a)
PackingGroup: II
3-(Methylamino)toluene Specification
3-(Methylamino)toluene (CAS NO.696-44-6) is also named as Aniline, N,m-dimethyl- ; N,3-Dimethylaniline ; N-3-Dimethylbenzenamine ; N-Methyl-3-methylaniline ; N-Methyl-m-toluidine ; NSC 9396 ; m,N-Dimethylaniline ; Benzenamine, N,3-dimethyl- . 3-(Methylamino)toluene (CAS NO.696-44-6) is clear yellow liquid.
Related Products
- 3-(Methylamino)toluene
- 6964-53-0
- 696-45-7
- 69645-84-7
- 69646-14-6
- 6964-62-1
- 6964-81-4
- 6964-98-3
- 69651-80-5
- 6965-32-8
- 696-54-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View