-
Name
3,3,4,4,5,5,6,6,7,7,8,8,8-Tridecafluoro-1-octanol
- EINECS 211-477-1
- CAS No. 647-42-7
- Article Data31
- CAS DataBase
- Density 1.589 g/cm3
- Solubility Insoluble in water
- Melting Point
- Formula C8H5F13O
- Boiling Point 174.1 °C at 760 mmHg
- Molecular Weight 364.106
- Flash Point 91.667 °C
- Transport Information
- Appearance Colorless liquid
- Safety 26-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 1,1,2,2-Tetrahydroperfluorooctan-1-ol;1,1,2,2-Tetrahydroperfluorooctanol;1,1,2,2-Tetrahydrotridecafluorooctanol;1H,1H,2H,2H-Perfluoro-1-octanol;1H,1H,2H,2H-Perfluorooctan-1-ol;1H,1H,2H,2H-Perfluorooctanol;1H,1H,2H,2H-Tridecafluoro-n-octanol;1H,1H,2H,2H-Tridecafluorooctanol;2-(Perfluorohexyl)ethanol;2-(Perfluorohexyl)ethyl alcohol;2-(Tridecafluorohexyl)ethanol;1-Octanol,3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluoro-;A 1620;Fluowet EA 600;Foralkyl EOH 6;Perfluorohexylethanol;
- PSA 20.23000
- LogP 4.10760
Synthetic route
-
-
2043-57-4
1,1,1,2,2,3,3,4,4,5,5,6,6-Tridecafluoro-8-iodooctane

-
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

| Conditions | Yield |
|---|---|
| With hydrogenchloride; formic acid; oxygen; copper; zinc In water at 80℃; for 2h; Product distribution; various solvents, water concentration; | 100% |
| With water In N,N-dimethyl-formamide at 125 - 130℃; under 2625.26 Torr; for 32h; Large scale; Green chemistry; | 88% |
| Yield given. Multistep reaction; |
-
-
1262446-12-7
potassium 8,8,8,7,7,6,6,5,5,4,4,3,3-tridecafluorooctyl sulfate

-
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid; water at 20℃; for 0.166667h; | 96% |

-
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; sodium hydroxide In water at 45℃; for 2h; Temperature; | 91% |
-
-
155939-24-5
3,4-Dihydro-5-<(3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluoro)octyloxy>-2H-pyrrole

-
A
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

-
B
-
25291-17-2
3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooct-1-ene

| Conditions | Yield |
|---|---|
| With 2-pyrrolidinon at 140℃; for 3h; | A 90% B 1% |
-
-
830330-61-5
3-(2-fluoro-phenyl)-3-hydroxy-propionic acid 3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluoro-octyl ester

-
A
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

-
B
-
91319-56-1
3-hydroxy-3-(2'-fluorophenyl)propanol

| Conditions | Yield |
|---|---|
| With lithium triethylborohydride In tetrahydrofuran at 20℃; for 2h; | A n/a B 88% |
-
-
2043-57-4
1,1,1,2,2,3,3,4,4,5,5,6,6-Tridecafluoro-8-iodooctane

-
A
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

-
B
-
1741-03-3
(C6F13C2H4O)2SO2

| Conditions | Yield |
|---|---|
| Stage #1: 1,1,1,2,2,3,3,4,4,5,5,6,6-Tridecafluoro-8-iodooctane With sulfuric acid; sulfur trioxide In water at 30℃; for 1.66h; Stage #2: With sodium sulfite; water at 0 - 80℃; for 2h; Product distribution / selectivity; | A 82.3% B n/a |
| Stage #1: 1,1,1,2,2,3,3,4,4,5,5,6,6-Tridecafluoro-8-iodooctane With sulfuric acid; sulfur trioxide at 20℃; for 1.66h; Stage #2: With sodium sulfite; water at 20 - 80℃; for 2h; Product distribution / selectivity; | A 81.1% B n/a |
-
-
2043-57-4
1,1,1,2,2,3,3,4,4,5,5,6,6-Tridecafluoro-8-iodooctane

-
-
1741-03-3
(C6F13C2H4O)2SO2

-
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

| Conditions | Yield |
|---|---|
| Stage #1: 1,1,1,2,2,3,3,4,4,5,5,6,6-Tridecafluoro-8-iodooctane; (C6F13C2H4O)2SO2 With sulfuric acid; sulfur trioxide at 20 - 80℃; for 1.66h; Stage #2: With sodium sulfite; water at 20 - 80℃; for 2h; Product distribution / selectivity; | 75.9% |

| Conditions | Yield |
|---|---|
| Stage #1: (C6F13C2H4O)2SO2 With sulfuric acid; sulfur trioxide at 20 - 80℃; for 1.33h; Stage #2: With water at 20 - 80℃; for 1.5 - 2h; Product distribution / selectivity; | 70.3% |
-
-
2043-57-4
1,1,1,2,2,3,3,4,4,5,5,6,6-Tridecafluoro-8-iodooctane

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
A
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

-
B
-
51066-74-1
dimethylamine hydriodide

| Conditions | Yield |
|---|---|
| With water Product distribution; | A 33% B n/a C 66% |

-
-
830330-63-7
3-hydroxy-3-phenyl-propionic acid 3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluoro-octyl ester

-
A
-
4850-49-1
1-phenyl-1,3-propanediol

-
B
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

| Conditions | Yield |
|---|---|
| With lithium triethylborohydride In tetrahydrofuran at 20℃; for 2h; | A 61% B n/a |
-
-
830330-65-9
3-hydroxy-3-o-tolyl-propionic acid 3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluoro-octyl ester

-
A
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

| Conditions | Yield |
|---|---|
| With lithium triethylborohydride In tetrahydrofuran at 20℃; for 2h; | A n/a B 55% |
-
-
4938-92-5
N-(2-formylaminoethyl)formamide

-
-
2043-57-4
1,1,1,2,2,3,3,4,4,5,5,6,6-Tridecafluoro-8-iodooctane

-
A
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

-
B
-
25291-17-2
3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooct-1-ene

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 150℃; for 24h; | A 31.4% B 16.7% C 51% |
| In N,N-dimethyl-formamide at 150℃; for 24h; | A 31.4% B 16.7% C 51% |

-
-
675-20-7
piperidin-2-one

-
-
2043-57-4
1,1,1,2,2,3,3,4,4,5,5,6,6-Tridecafluoro-8-iodooctane

-
A
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

-
B
-
25291-17-2
3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooct-1-ene

| Conditions | Yield |
|---|---|
| at 140℃; for 7.25h; Yields of byproduct given; | A n/a B n/a C 18.3% |

-
-
2043-57-4
1,1,1,2,2,3,3,4,4,5,5,6,6-Tridecafluoro-8-iodooctane

-
A
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

| Conditions | Yield |
|---|---|
| With sodium azide In N,N-dimethyl-formamide for 12h; Reflux; | A n/a B 15% |
-
-
123-39-7
N-Methylformamide

-
-
2043-57-4
1,1,1,2,2,3,3,4,4,5,5,6,6-Tridecafluoro-8-iodooctane

-
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

| Conditions | Yield |
|---|---|
| With sodium hydroxide; water 1.) 140 degC, 6 h; 2.)62-85 degC, 22 h; Yield given. Multistep reaction; |
-
-
123-39-7
N-Methylformamide

-
-
2043-57-4
1,1,1,2,2,3,3,4,4,5,5,6,6-Tridecafluoro-8-iodooctane

-
A
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

-
B
-
25291-17-2
3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooct-1-ene

| Conditions | Yield |
|---|---|
| at 140℃; for 1h; Product distribution; Rate constant; var. temp., time, substrate (DMF) investigated; |

-
-
123-39-7
N-Methylformamide

-
-
2043-57-4
1,1,1,2,2,3,3,4,4,5,5,6,6-Tridecafluoro-8-iodooctane

-
A
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

| Conditions | Yield |
|---|---|
| Yield given. Yields of byproduct given; |

-
-
16741-46-1
N-isopropylformamide

-
-
2043-57-4
1,1,1,2,2,3,3,4,4,5,5,6,6-Tridecafluoro-8-iodooctane

-
A
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

-
B
-
25291-17-2
3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooct-1-ene

| Conditions | Yield |
|---|---|
| With water at 140℃; for 1h; Product distribution; Rate constant; var. temp., time, substrate (DMF, 1,2-ethanyl-bis-formamide) investigated; |

-
-
2043-57-4
1,1,1,2,2,3,3,4,4,5,5,6,6-Tridecafluoro-8-iodooctane

-
A
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

-
B
-
25291-17-2
3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooct-1-ene

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol at 30℃; Product distribution; variation of condition; also with NaOMe; | |
| With radical initiator; water In N,N-dimethyl-formamide Heating; | |
| With water; lithium chloride In N,N-dimethyl-formamide for 69h; electrolysis at carbon fibre cathode; | A 68 % Spectr. B 32 % Spectr. |
-
-
2043-57-4
1,1,1,2,2,3,3,4,4,5,5,6,6-Tridecafluoro-8-iodooctane

-
A
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

-
B
-
25291-17-2
3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooct-1-ene

| Conditions | Yield |
|---|---|
| With water; formamide at 150℃; for 6h; | A 5.2 % Chromat. B 1.4 % Chromat. C n/a D 7.2 % Chromat. |
| With water; formamide at 140℃; for 4.5h; | A 3.9 % Chromat. B 3.9 % Chromat. C n/a D 10.0 % Chromat. |

-
-
2043-57-4
1,1,1,2,2,3,3,4,4,5,5,6,6-Tridecafluoro-8-iodooctane

-
A
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

-
B
-
25291-17-2
3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooct-1-ene

-
C
-
80793-17-5
(perfluoro-n-hexyl)ethane

| Conditions | Yield |
|---|---|
| With water; lithium chloride In N,N-dimethyl-formamide Product distribution; effect of quantity of water and current on electroreduction; | A 80 % Spectr. B 5 % Spectr. C 15 % Spectr. |

-
-
2043-57-4
1,1,1,2,2,3,3,4,4,5,5,6,6-Tridecafluoro-8-iodooctane

-
A
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

| Conditions | Yield |
|---|---|
| With N-Methylformamide Yield given. Yields of byproduct given; |
-
-
2043-57-4
1,1,1,2,2,3,3,4,4,5,5,6,6-Tridecafluoro-8-iodooctane

-
-
77287-34-4, 77287-35-5, 60100-09-6
formamide

-
A
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

-
B
-
25291-17-2
3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooct-1-ene

| Conditions | Yield |
|---|---|
| With water at 140℃; for 4.5h; | A 3.9 % Chromat. B 3.9 % Chromat. C n/a D 10.0 % Chromat. |

-
-
77287-34-4, 77287-35-5, 60100-09-6
formamide

-
A
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

-
B
-
25291-17-2
3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooct-1-ene

| Conditions | Yield |
|---|---|
| With 2-(F-hexyl)-1-iodoethane; water at 140℃; for 4.5h; | A 3.9 % Chromat. B 3.9 % Chromat. C n/a D 10.0 % Chromat. |

-
-
78522-69-7
3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooctyl trifluoromethanesulfonate

-
A
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

-
B
-
25291-17-2
3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooct-1-ene

-
C
-
78522-74-4
1,1,1,2,2,3,3,4,4,5,5,6,6-Tridecafluoro-8-(3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluoro-octyloxy)-octane

| Conditions | Yield |
|---|---|
| With solution aqueuse de soude 10 N for 0.25h; Heating; | A 33 % Spectr. B 31 % Spectr. C 35 % Spectr. |

-
-
616-45-5
2-pyrrolidinon

-
-
2043-57-4
1,1,1,2,2,3,3,4,4,5,5,6,6-Tridecafluoro-8-iodooctane

-
A
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

-
B
-
25291-17-2
3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooct-1-ene

| Conditions | Yield |
|---|---|
| at 140℃; for 1h; Product distribution; Mechanism; Rate constant; other times, temperatures; also with ε-caprolactam; |

-
-
616-45-5
2-pyrrolidinon

-
-
2043-57-4
1,1,1,2,2,3,3,4,4,5,5,6,6-Tridecafluoro-8-iodooctane

-
A
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

-
B
-
7060-52-8
1-(1-pyrrolidin-2-yl)-2-pyrrolidone

| Conditions | Yield |
|---|---|
| With potassium carbonate 1.) 140 deg C, 6.00 h, 2.) EtOH, CHCl3, 68 deg C, 5 h; Yield given. Multistep reaction. Yields of byproduct given; |

-
-
616-45-5
2-pyrrolidinon

-
-
155939-24-5
3,4-Dihydro-5-<(3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluoro)octyloxy>-2H-pyrrole

-
A
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

-
B
-
7060-52-8
1-(1-pyrrolidin-2-yl)-2-pyrrolidone

| Conditions | Yield |
|---|---|
| In toluene at 140℃; Rate constant; also without solvent at other temperatures; |

-
-
616-45-5
2-pyrrolidinon

-
-
155939-25-6
3,4,5,6-Tetrahydro-7-<2-(3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluoro)octyloxy>-2H-azepine

-
A
-
105-60-2
caprolactam

-
B
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

-
C
-
25291-17-2
3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooct-1-ene

-
D
-
90683-34-4
1-(4,5,6,7-tetrahydro-3H-azepin-2-yl)-2-pyrrolidinone

| Conditions | Yield |
|---|---|
| In toluene at 140℃; for 24h; Yields of byproduct given. Title compound not separated from byproducts; | A n/a B n/a C n/a D 47.4 % Chromat. |
| In toluene at 140℃; Rate constant; |

-
-
675-20-7
piperidin-2-one

-
-
2043-57-4
1,1,1,2,2,3,3,4,4,5,5,6,6-Tridecafluoro-8-iodooctane

-
A
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

-
B
-
25291-17-2
3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooct-1-ene

| Conditions | Yield |
|---|---|
| at 140℃; for 1h; Rate constant; Mechanism; other times; also with ε-caprolactam; |

-
-
75-44-5
phosgene

-
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

-
-
181302-91-0
3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooctyl chloroformate

| Conditions | Yield |
|---|---|
| In diethyl ether for 24h; Heating; | 100% |

| Conditions | Yield |
|---|---|
| With bismuth(lll) trifluoromethanesulfonate In 1,2-dichloro-ethane at 80℃; for 24h; Inert atmosphere; Schlenk technique; chemoselective reaction; | 100% |

| Conditions | Yield |
|---|---|
| With 1,10-Phenanthroline; palladium diacetate at 45℃; for 96h; Time; Temperature; | 98.2% |
-
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

-
-
53826-12-3
3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooctanoic acid

| Conditions | Yield |
|---|---|
| With chromium(VI) oxide; sulfuric acid In diethyl ether; acetone for 0.0833333h; | 98% |
| Stage #1: 1H,1H,2H,2H-tridecafluoro-n-octanol With Jones reagent In acetone Stage #2: With isopropyl alcohol In acetone | 84% |
| With chromium(VI) oxide; sulfuric acid In diethyl ether; water; acetone for 0.5h; Jones oxidation; | 76% |
-
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

-
-
22959-55-3
β,β,β-trichloroethoxysulfonyl isocyanate

| Conditions | Yield |
|---|---|
| at 20℃; for 0.0166667h; Addition; | 98% |
-
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

-
-
73748-48-8
<(4-nitrophenyl)oxy>sulfonyl isocyanate

| Conditions | Yield |
|---|---|
| at 20℃; for 0.0166667h; Addition; | 98% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid at 140℃; for 6h; | 98% |
-
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

-
-
292638-85-8
acrylic acid methyl ester

-
-
17527-29-6
3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooctyl 2-propenoate

| Conditions | Yield |
|---|---|
| With C32H16F52O4Ti; hydroquinone In toluene at 80℃; for 3.5h; Reagent/catalyst; Large scale; | 97.2% |
| With titanium(III) sulphate; hydroquinone In cyclohexane at 120℃; for 8h; Reagent/catalyst; |
-
-
74-96-4
ethyl bromide

-
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

-
-
210896-77-8
1,1,1,2,2,3,3,4,4,5,5,6,6-tridecafluoro-9-oxaundecane

| Conditions | Yield |
|---|---|
| With potassium hydroxide In 1-methyl-pyrrolidin-2-one; water at 50 - 70℃; for 7h; | 97% |
-
-
108-30-5
succinic acid anhydride

-
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

-
-
125111-32-2
1H,2H,2H-perfluoro-1-octyl succinic acid monoester

| Conditions | Yield |
|---|---|
| With dmap In tetrahydrofuran at 100℃; for 2h; | 97% |
| With triethylamine In tetrahydrofuran at 100℃; for 1h; | 94% |
| In tetrahydrofuran at 100℃; for 1h; | 94% |
| With triethylamine; N-ethyl-N,N-diisopropylamine | |
| With pyridine In 1,2-dimethoxyethane at 65 - 75℃; for 36h; Inert atmosphere; |
-
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

-
-
134052-00-9
3,3,4,4,5,5,6,6,7,7,8,8,8-Tridecafluoro-octyl phosphorodichloridate

| Conditions | Yield |
|---|---|
| With triethylamine; trichlorophosphate In diethyl ether for 1h; Ambient temperature; | 96% |
| With triethylamine; trichlorophosphate In diethyl ether at 0℃; |

| Conditions | Yield |
|---|---|
| Pd-C In hydrogen | 96% |
-
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

-
-
104-15-4
toluene-4-sulfonic acid

-
-
56-40-6
glycine

-
-
911217-61-3
glycine 2-perfluorohexylethyl ester p-toluenesulphonate

| Conditions | Yield |
|---|---|
| In toluene for 4h; Heating; | 96% |
| In cyclohexane for 17h; Reflux; | 78% |


| Conditions | Yield |
|---|---|
| With saccharin sodium salt at 80 - 90℃; for 2h; | 95.5% |
-
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

-
-
107-05-1
3-chloroprop-1-ene

-
-
103628-86-0
8-(allyloxyl)-1,1,1,2,2,3,3,4,4,5,5,6,6-tridecafluorooctane

| Conditions | Yield |
|---|---|
| With sodium hydroxide; tetra(n-butyl)ammonium hydroxide at 40℃; for 6h; | 95% |
| With sodium hydroxide; tetra(n-butyl)ammonium hydrogensulfate at 45℃; for 6h; | 94% |
| With sodium hydroxide; tetra(n-butyl)ammonium hydrogensulfate at 30℃; for 8h; Yield given; |
-
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

-
-
14793-41-0
isocyanate de chloro-4 phenoxysulfonyle

| Conditions | Yield |
|---|---|
| at 20℃; for 0.0166667h; Addition; | 95% |

-
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

| Conditions | Yield |
|---|---|
| Pd-C In hydrogen | 95% |
| With hydrogen; palladium on activated charcoal at 160℃; for 10h; atmospheric pressure; | 92% |
-
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

| Conditions | Yield |
|---|---|
| With tetraphosphorus decasulfide In toluene for 12h; Heating; | 95% |
-
-
876-08-4
p-(chloromethyl)benzoyl chloride

-
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

-
-
1293408-03-3
C16H10ClF13O2

| Conditions | Yield |
|---|---|
| With triethylamine In acetone at 10 - 20℃; for 2h; Inert atmosphere; | 95% |
| With triethylamine In acetone at 10 - 20℃; for 2h; Inert atmosphere; | 95% |
-
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

| Conditions | Yield |
|---|---|
| With phosphorus pentoxide; phosphoric acid In water at 50 - 60℃; for 48h; Temperature; | 94.7% |
-
-
79-41-4
poly(methacrylic acid)

-
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

-
-
2144-53-8
3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooctyl 2-methylpropenoate

| Conditions | Yield |
|---|---|
| With hydroquinone; toluene-4-sulfonic acid In toluene at 115℃; for 5h; | 94% |
| With sulfuric acid; hydroquinone In benzene Heating; | |
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; |
-
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
51740-38-6
toluene-4-sulfonic acid 3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooctyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; | 94% |
| With triethylamine In dichloromethane | 92% |
| With triethylamine In dichloromethane at 0 - 20℃; Tosylation; | 91% |
-
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); triethylamine; 1,4-di(diphenylphosphino)-butane In dichloromethane at 60℃; for 48h; Inert atmosphere; Schlenk technique; stereoselective reaction; | 94% |
-
-
2525-16-8
O-methylcaprolactim

-
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

-
-
155939-25-6
3,4,5,6-Tetrahydro-7-<2-(3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluoro)octyloxy>-2H-azepine

| Conditions | Yield |
|---|---|
| Heating; | 93.4% |
-
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

-
-
106-95-6
allyl bromide

-
-
103628-86-0
8-(allyloxyl)-1,1,1,2,2,3,3,4,4,5,5,6,6-tridecafluorooctane

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 60℃; under 75.0075 Torr; for 1h; Temperature; Inert atmosphere; Large scale; | 93% |
| With sodium hydroxide at 60 - 80℃; for 4h; Inert atmosphere; | 88% |
| With sodium hydroxide at 60 - 80℃; for 4h; Inert atmosphere; | 88% |
-
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

-
-
598-21-0
2-Bromoacetyl bromide

-
-
132711-05-8
Bromure d'acetate de 2-F-hexylethyle

| Conditions | Yield |
|---|---|
| In diethyl ether at 0 - 20℃; for 12h; | 93% |
| In diethyl ether at 60℃; for 12h; | 68% |
| at 60℃; |
-
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol

| Conditions | Yield |
|---|---|
| Stage #1: 1H,1H,2H,2H-tridecafluoro-n-octanol With pyridine; di(succinimido) carbonate In N,N-dimethyl-formamide at 40℃; for 15h; Stage #2: Nα-(tert-butyloxycarbonyl)-(R)-lysine tert-butyl ester In N,N-dimethyl-formamide at 20 - 30℃; | 93% |

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal at 105℃; for 8h; | 92% |
| With hydrogen; palladium on activated charcoal at 105℃; for 8h; atmospheric pressure; | 92% |
3,3,4,4,5,5,6,6,7,7,8,8,8-Tridecafluoro-1-octanol Specification
3,3,4,4,5,5,6,6,7,7,8,8,8-Tridecafluoro-1-octanol is an organic compound with the formula C8H5F13O, and its systematic name is the same with the product name. With the CAS registry number 647-42-7, it is also named as 1H,1H,2H,2H-Perfluorooctan-1-ol. It belongs to the product categories of Organics; Organofluorine compounds. Its EINECS number is 211-477-1. In addition, the molecular weight is 364.11. This chemical should be sealed and stored in a cool and dry place. Moreover, it should be protected from oxides.And it is irritating to eyes, respiratory system and skin. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. When using it, you need wear suitable protective gloves and eye/face protection.
Physical properties of 3,3,4,4,5,5,6,6,7,7,8,8,8-Tridecafluoro-1-octanol are:
(1)ACD/LogP: 4.076; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 4.08; (4)ACD/LogD (pH 7.4): 4.08; (5)ACD/BCF (pH 5.5): 737.21; (6)ACD/BCF (pH 7.4): 737.21; (7)ACD/KOC (pH 5.5): 3928.48; (8)ACD/KOC (pH 7.4): 3928.48; (9)#H bond acceptors: 1; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 7; (12)Polar Surface Area: 20.23 Å2; (13)Index of Refraction: 1.298; (14)Molar Refractivity: 42.57 cm3; (15)Molar Volume: 229.205 cm3; (16)Polarizability: 16.876×10-24cm3; (17)Surface Tension: 16.4 dyne/cm; (18)Density: 1.589 g/cm3; (19)Flash Point: 91.667 °C; (20)Enthalpy of Vaporization: 47.786 kJ/mol; (21)Boiling Point: 174.1 °C at 760 mmHg; (22)Vapour Pressure: 0.38 mmHg at 25°C.
Preparation of 3,3,4,4,5,5,6,6,7,7,8,8,8-Tridecafluoro-1-octanol:
The 3,3,4,4,5,5,6,6,7,7,8,8,8-Tridecafluoro-1-octanol can be prepared by 7H,7H,8H,8H,8H-tridecafluoro-8-iodo-octane at the temperature of 80 °C. This reaction will need reagents Zn/Cu, O2, formic acid - sodium formate, 20% HCl and solvents various solvent(s), H2O with the reaction time of 2 hours. The yield is about 100%.
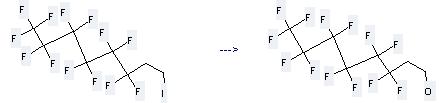
Uses of 3,3,4,4,5,5,6,6,7,7,8,8,8-Tridecafluoro-1-octanol:
it can be used to produce 2-(3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluoro-octyloxy)-ethanol at the temperature of 60 °C. It will need reagent triethylamine and solvent bis-(2-methoxy-ethyl) ether with the reaction time of 12 hours. The yield is about 76%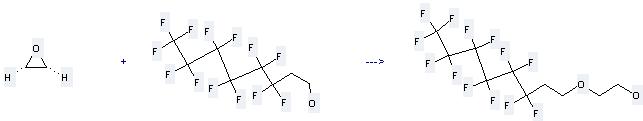
You can still convert the following datas into molecular structure:
(1)SMILES: FC(F)(C(F)(F)CCO)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F
(2)Std. InChI: InChI=1S/C8H5F13O/c9-3(10,1-2-22)4(11,12)5(13,14)6(15,16)7(17,18)8(19,20)21/h22H,1-2H2
(3)Std. InChIKey: GRJRKPMIRMSBNK-UHFFFAOYSA-N
Related Products
- 3,10-Diaminotricyclo(5.2.1.0(sup 2,6))decane
- 3,10-Dinitrophenanthrene
- 3-((10-ETHYL-11-(p-HYDROXYPHENYL)DIBENZ-(B,F)OXEPIN-3-YL)OXY)-1,2-PROPANEDIOL HYDRATE (4:1)
- 3-(1,1,2,2-Tetrafluoroethoxy)aniline
- 3-(1,1,2,2-Tetrafluoroethoxy)benzaldehyde
- 3-(1,1,2,2-Tetrafluoroethoxy)bromobenzene
- 3-(1,1,2,2-Tetrafluoroethoxy)toluene
- 3-[1,1'-Biphenyl]-4-yl-1,2,3,4-tetrahydro-1-naphthol
- 3,11-Dichloro-6,11-dihydro-6-methyldibenzo[c,f][1,2]thiazepine 5,5-dioxide
- 3-[(1,1-Dimethyl-2-hydroxyethyl)amino]-2-hydroxypropanesulfonicacid
- 64742-79-6
- 64742-81-0
- 64742-82-1
- 64742-88-7
- 64742-89-8
- 64742-90-1
- 64742-93-4
- 64742-94-5
- 64742-95-6
- 64743-02-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View