-
Name
3,5-Dimethoxybenzaldehyde
- EINECS 230-772-6
- CAS No. 7311-34-4
- Article Data119
- CAS DataBase
- Density 1.114 g/cm3
- Solubility insoluble in water
- Melting Point 45-48 °C(lit.)
- Formula C9H10O3
- Boiling Point 276.5 °C at 760 mmHg
- Molecular Weight 166.177
- Flash Point 119 °C
- Transport Information
- Appearance white to beige crystalline solid
- Safety 26-36-24/25
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Benzaldehyde, 3,5-dimethoxy-;
- PSA 35.53000
- LogP 1.51630
Synthetic route
-
-
34967-25-4
3,5-dimethoxybenzaldoxime

-
-
7311-34-4
3,5-dimethoxybenzaldehdye

| Conditions | Yield |
|---|---|
| With benzyltriphenylphosphonium dichromate In acetonitrile for 0.25h; Oxidation; Heating; | 100% |
| With 1,4-dichloro-1,4-diazoniabicyclo[2,2,2]octane bischloride In water at 50℃; for 0.333333h; pH=7; | 85% |
| With quinolinium chlorochromate(VI) for 0.583333h; | 50% |

| Conditions | Yield |
|---|---|
| With silica-supported Jones reagent In dichloromethane for 0.00269444h; | 99.5% |
| With 1,2-dimethyl-3-[6-(methylsulfinyl)hexyl]-1H-imidazolium triflate; oxalyl dichloride; triethylamine In dichloromethane; acetonitrile at -78 - 20℃; Swern oxidation; | 97% |
| With Dess-Martin periodane In dichloromethane at 0℃; Inert atmosphere; | 96% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 50℃; | 99% |
| With potassium carbonate In acetone at 80℃; for 12h; Inert atmosphere; Schlenk technique; | 92% |
| With potassium carbonate In acetone Heating; | 90% |
| Stage #1: 3,5-dihydroxybenzaldehyde With potassium carbonate In acetone at 20℃; for 0.5h; Inert atmosphere; Stage #2: methyl iodide In acetone for 6h; Reflux; Inert atmosphere; | 69.4% |
-
-
74726-76-4
2-bromo-3,5-dimethoxybenzyl alcohol

-
-
7311-34-4
3,5-dimethoxybenzaldehdye

| Conditions | Yield |
|---|---|
| Stage #1: 2-bromo-3,5-dimethoxybenzyl alcohol With water; lead(IV) tetraacetate; potassium nitrate at 37℃; for 1.5h; Stage #2: With rhenium(IV) sulfide at 45℃; for 2h; Temperature; | 98.5% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In chloroform at 20℃; for 24h; Irradiation; Inert atmosphere; | 96% |
| With dipotassium peroxodisulfate In water at 90℃; for 12h; Green chemistry; | 62% |

| Conditions | Yield |
|---|---|
| With nickel-doped graphene carbon nitride nanoparticles; air In ethanol at 25℃; for 8h; Irradiation; Green chemistry; | 95% |
| Multi-step reaction with 2 steps 1: N-Bromosuccinimide / acetonitrile / 1 h / 25 °C / Inert atmosphere 2: hexamethylenetetramine / water / 12 h / 120 °C / Inert atmosphere View Scheme |
-
-
15226-74-1, 61091-28-9, 61117-58-6
dicobalt octacarbonyl

-
-
20469-65-2
1-bromo-3,5-dimethoxybenzene

-
-
7311-34-4
3,5-dimethoxybenzaldehdye

| Conditions | Yield |
|---|---|
| With triethylsilane; dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; sodium acetate In acetonitrile at 25℃; for 16h; Schlenk technique; Inert atmosphere; | 87% |

| Conditions | Yield |
|---|---|
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In dichloromethane; water for 2.5h; Ambient temperature; | A n/a B 86% |

-
A
-
7311-34-4
3,5-dimethoxybenzaldehdye

-
B
-
96016-38-5
(3R,4S)-6-Benzyloxy-4-(4-methoxy-benzyloxymethyl)-hexan-3-ol

| Conditions | Yield |
|---|---|
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In water; Petroleum ether at 5℃; for 0.08h; | A n/a B 86% |

-
A
-
7311-34-4
3,5-dimethoxybenzaldehdye

-
B
-
96016-18-1
(2R,3S,4S)-3,5-Bis-benzyloxy-4-methyl-pentan-2-ol

| Conditions | Yield |
|---|---|
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In dichloromethane; water for 0.25h; Ambient temperature; | A n/a B 85% |

-
A
-
7311-34-4
3,5-dimethoxybenzaldehdye

-
B
-
123-11-5
4-methoxy-benzaldehyde

-
D
-
96016-37-4, 107297-50-7
(2R,3R,4R)-4-(4-Methoxy-benzyloxy)-3,5-bis-methoxymethoxy-pentan-2-ol

| Conditions | Yield |
|---|---|
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In water; Petroleum ether at 5℃; for 0.6h; | A n/a B n/a C 4% D 80% |
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In water; Petroleum ether at 5℃; for 0.6h; | A n/a B n/a C 4% D 4% |

-
-
20469-65-2
1-bromo-3,5-dimethoxybenzene

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
-
7311-34-4
3,5-dimethoxybenzaldehdye

| Conditions | Yield |
|---|---|
| Stage #1: 1-bromo-3,5-dimethoxybenzene With n-butyllithium In tetrahydrofuran; hexane for 0.333333h; Inert atmosphere; Cooling; Stage #2: N,N-dimethyl-formamide In tetrahydrofuran; hexane for 0.333333h; Cooling; Inert atmosphere; | 78% |

| Conditions | Yield |
|---|---|
| With iodine; triethylamine; triphenylphosphine In toluene at 80℃; Inert atmosphere; Sealed tube; | 77% |
-
-
712329-47-0
1-(azidomethyl)-3,5-dimethoxybenzene

-
-
7311-34-4
3,5-dimethoxybenzaldehdye

| Conditions | Yield |
|---|---|
| Stage #1: 1-(azidomethyl)-3,5-dimethoxybenzene With sodium hydride In dimethyl sulfoxide; mineral oil at 20℃; for 4h; Inert atmosphere; Stage #2: With water In dimethyl sulfoxide; mineral oil for 0.25h; Inert atmosphere; | 76% |

-
A
-
7311-34-4
3,5-dimethoxybenzaldehdye

-
B
-
123-11-5
4-methoxy-benzaldehyde

-
D
-
96016-36-3
(2R,3R)-2-Benzyloxy-4-(4-methoxy-benzyloxy)-3-methoxymethoxy-butan-1-ol

| Conditions | Yield |
|---|---|
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In dichloromethane; water at 5℃; for 1.8h; | A n/a B n/a C 6.5% D 75% |
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In dichloromethane; water at 5℃; for 1.8h; | A n/a B n/a C 6.5% D 6.5% |


| Conditions | Yield |
|---|---|
| With quinoline; (1,3-dimesitylimidazolidin-2-yl)copper(I) chloride; oxygen In 1,4-dioxane at 100℃; under 760.051 Torr; for 24h; | A 74% B 61% |
-
-
22255-22-7
3,5,4'-trimethoxy-trans-stilbene

-
A
-
7311-34-4
3,5-dimethoxybenzaldehdye

-
B
-
123-11-5
4-methoxy-benzaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: 3,5,4'-trimethoxy-trans-stilbene With ozone In methanol; dichloromethane at -78℃; Stage #2: With triphenylphosphine In methanol; dichloromethane for 0.5h; | A 60% B 73% |
-
-
17213-57-9
3,5-dimethoxybenzoyl chloride

-
-
7311-34-4
3,5-dimethoxybenzaldehdye

| Conditions | Yield |
|---|---|
| With hydrogen; sodium acetate; palladium on activated charcoal under 2427.2 Torr; 1h, then 60 degC, overnight; | 70% |
| With tri-n-butyl-tin hydride In 1-methyl-pyrrolidin-2-one at 20℃; Inert atmosphere; | 60% |
| With Pd-BaSO4; xylene at 140℃; Hydrogenation; |

| Conditions | Yield |
|---|---|
| With triethylsilane; dichloro bis(acetonitrile) palladium(II); sodium carbonate; 1,4-di(diphenylphosphino)-butane In N,N-dimethyl-formamide at 20 - 100℃; under 15001.5 Torr; for 20h; Inert atmosphere; Autoclave; | 70% |

| Conditions | Yield |
|---|---|
| With quinoline; (1,3-dimesitylimidazolidin-2-yl)copper(I) chloride; oxygen In 1,4-dioxane at 100℃; under 760.051 Torr; for 24h; | A 70% B 67% |

-
A
-
7311-34-4
3,5-dimethoxybenzaldehdye

-
B
-
96016-44-3, 107297-53-0
[(4S,5R)-5-Ethyl-2-(4-methoxy-phenyl)-4-methyl-[1,3]dioxolan-4-yl]-methanol

-
C
-
96016-45-4
4-Methoxy-benzoic acid (1R,2S)-1-ethyl-2,3-dihydroxy-2-methyl-propyl ester

| Conditions | Yield |
|---|---|
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In water; Petroleum ether at 10℃; for 1h; | A n/a B 66% C 5.5% |


| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In methanol at 20℃; for 0.166667h; | 100% |
| With sodium tetrahydroborate In methanol at 20℃; for 1h; | 100% |
| With sodium tetrahydroborate In ethanol at 20℃; for 0.5h; Inert atmosphere; | 100% |
-
-
5425-44-5
2-Phenyl-[1,3]dithiane

-
-
7311-34-4
3,5-dimethoxybenzaldehdye

-
-
92989-59-8
(3,5-dimethoxyphenyl)(2-phenyl-[1,3]-dithian-2-yl)-methanol

| Conditions | Yield |
|---|---|
| Stage #1: 2-Phenyl-[1,3]dithiane With n-butyllithium In tetrahydrofuran at 0℃; for 0.5h; Stage #2: 3,5-dimethoxybenzaldehdye In tetrahydrofuran at 0 - 20℃; for 1.5h; Further stages.; | 100% |
| Stage #1: 2-Phenyl-[1,3]dithiane With n-butyllithium In tetrahydrofuran at 0℃; Stage #2: 3,5-dimethoxybenzaldehdye In tetrahydrofuran at 0 - 20℃; for 1.5h; | 100% |
| Stage #1: 2-Phenyl-[1,3]dithiane With n-butyllithium In tetrahydrofuran; cyclohexane at -78℃; Inert atmosphere; Stage #2: 3,5-dimethoxybenzaldehdye In tetrahydrofuran; cyclohexane at -78 - 20℃; Inert atmosphere; | 90% |
-
-
558-13-4
carbon tetrabromide

-
-
7311-34-4
3,5-dimethoxybenzaldehdye

-
-
205746-51-6
1-(2,2-dibromoethen-1-yl)-3,5-dimethoxybenzene

| Conditions | Yield |
|---|---|
| With triphenylphosphine In dichloromethane at 0℃; for 2h; Inert atmosphere; | 100% |
| Stage #1: carbon tetrabromide With triphenylphosphine In dichloromethane at 0℃; for 0.5h; Inert atmosphere; Schlenk technique; Stage #2: 3,5-dimethoxybenzaldehdye In dichloromethane Inert atmosphere; Schlenk technique; | 97% |
| With triphenylphosphine In dichloromethane at 0 - 20℃; for 0.5h; | 96.4% |
-
-
2537-48-6
diethyl 1-cyanomethylphosphonate

-
-
7311-34-4
3,5-dimethoxybenzaldehdye

-
-
153507-02-9
3-(3,5-dimethoxyphenyl)acrylonitrile

| Conditions | Yield |
|---|---|
| Stage #1: diethyl 1-cyanomethylphosphonate With sodium hydride In tetrahydrofuran at 20℃; for 0.25h; Stage #2: 3,5-dimethoxybenzaldehdye In tetrahydrofuran at 20℃; for 17h; | 100% |
| Stage #1: diethyl 1-cyanomethylphosphonate With sodium hydride In tetrahydrofuran at 20℃; for 1h; Inert atmosphere; Stage #2: 3,5-dimethoxybenzaldehdye In tetrahydrofuran at 28℃; |
-
-
123-75-1
pyrrolidine

-
-
7677-24-9
trimethylsilyl cyanide

-
-
7311-34-4
3,5-dimethoxybenzaldehdye

-
-
1108730-66-0
2-(3,5-dimethoxyphenyl)-2-(pyrrolidin-1-yl)acetonitrile

| Conditions | Yield |
|---|---|
| With polymer-supported scandium(III) bis(trifluoromethanesulfonate) In acetonitrile at 20℃; for 0.5h; Strecker reaction; Combinatorial reaction / High throughput screening (HTS); chemoselective reaction; | 100% |

-
-
7677-24-9
trimethylsilyl cyanide

-
-
7311-34-4
3,5-dimethoxybenzaldehdye

-
-
2038-57-5
3-Phenylpropan-1-amine

-
-
1108730-65-9
2-(3,5-dimethoxyphenyl)-2-(3-phenylpropylamino)acetonitrile

| Conditions | Yield |
|---|---|
| With polymer-supported scandium(III) bis(trifluoromethanesulfonate) In acetonitrile at 20℃; for 1h; Strecker reaction; Combinatorial reaction / High throughput screening (HTS); chemoselective reaction; | 100% |

-
-
7311-34-4
3,5-dimethoxybenzaldehdye

-
-
121056-95-9
trimethyl 2-(tert-butoxycarbonylamino)phosphonoacetate

-
-
1259394-66-5
(Z)-methyl 2-[(tert-butoxycarbonyl)amino]-3-(3,5-dimethoxyphenyl)acrylate

| Conditions | Yield |
|---|---|
| With caesium carbonate for 7h; Horner-Wadsworth-Emmons olefination; neat (no solvent, solid phase); stereoselective reaction; | 100% |
-
-
870-46-2
t-butoxycarbonylhydrazine

-
-
7311-34-4
3,5-dimethoxybenzaldehdye

-
-
106728-65-8
(E)-tert-butyl 2-(3,5-dimethoxybenzylidene)hydrazinecarboxylate

| Conditions | Yield |
|---|---|
| at 20℃; for 1h; Neat (no solvent); Ball-milling; | 100% |
-
-
7311-34-4
3,5-dimethoxybenzaldehdye

-
-
613-94-5
benzoic acid hydrazide

-
-
678536-02-2
(E)-N'-(3,5-dimethoxybenzylidene)benzohydrazide

| Conditions | Yield |
|---|---|
| at 20℃; for 2h; Neat (no solvent); Ball-milling; | 100% |
| In ethanol Reflux; |
-
-
7311-34-4
3,5-dimethoxybenzaldehdye

-
-
1576-35-8
toluene-4-sulfonic acid hydrazide

-
-
1338091-83-0
(E)-N′-(3,5-dimethoxybenzylidene)-4-methylbenzenesulfonohydrazide

| Conditions | Yield |
|---|---|
| at 20℃; for 3h; Neat (no solvent); Ball-milling; | 100% |
| In methanol at 60℃; for 0.0833333 - 1h; | 95% |

| Conditions | Yield |
|---|---|
| In toluene for 20h; Reflux; Molecular sieve; | 100% |
-
-
867-13-0
diethoxyphosphoryl-acetic acid ethyl ester

-
-
7311-34-4
3,5-dimethoxybenzaldehdye

-
-
29584-64-3, 42174-79-8
ethyl 3,5-dimethoxycinnamate

| Conditions | Yield |
|---|---|
| With potassium carbonate In tetrahydrofuran for 20h; Horner-Wadsworth-Emmons reaction; Reflux; | 100% |
| With sodium tert-pentoxide at 0 - 20℃; for 1h; Horner-Wadsworth-Emmons Olefination; Green chemistry; |
-
-
7311-34-4
3,5-dimethoxybenzaldehdye

-
-
540-63-6
ethane-1,2-dithiol

-
-
61571-96-8
2-(3,5-dimethoxy-phenyl)-1,3-dithiolane

| Conditions | Yield |
|---|---|
| With diethyl ether; boron trifluoride In dichloromethane for 15h; | 99% |
| With amberlyst-15 In acetonitrile for 1h; | 99.93% |
| With boron trifluoride diethyl etherate In dichloromethane at 0℃; for 2h; |
-
-
109-80-8
1.3-propanedithiol

-
-
7311-34-4
3,5-dimethoxybenzaldehdye

-
-
57009-72-0
2-(3,5-dimethoxy-phenyl)-1,3-dithiane

| Conditions | Yield |
|---|---|
| With amberlyst-15 In acetonitrile for 1h; | 99.91% |
| With nickel dichloride In methanol; dichloromethane at 20℃; Inert atmosphere; | 90% |
| With hydrogenchloride In chloroform for 0.583333h; | 78% |
| With hydrogenchloride In chloroform for 18h; Ambient temperature; | 75.8% |

| Conditions | Yield |
|---|---|
| With piperazine In acetonitrile for 1h; Knoevenagel condensation; electroosmos; | 99.9% |
-
-
7677-24-9
trimethylsilyl cyanide

-
-
7311-34-4
3,5-dimethoxybenzaldehdye

-
-
64-04-0
phenethylamine

-
-
1108730-64-8
2-(3,5-dimethoxyphenyl)-2-(phenethylamino)acetonitrile

| Conditions | Yield |
|---|---|
| With polymer-supported scandium(III) bis(trifluoromethanesulfonate) In acetonitrile at 20℃; for 1h; Strecker reaction; Combinatorial reaction / High throughput screening (HTS); chemoselective reaction; | 99.9% |


| Conditions | Yield |
|---|---|
| With polymer-supported scandium(III) bis(trifluoromethanesulfonate) In acetonitrile at 20℃; for 1h; Strecker reaction; Combinatorial reaction / High throughput screening (HTS); chemoselective reaction; | 99.9% |

-
-
7311-34-4
3,5-dimethoxybenzaldehdye

-
-
105-56-6
ethyl 2-cyanoacetate

-
-
5864-90-4
3-(3,5-dimethoxyphenyl)-2-cyano-acrylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With 3-(1-piperazino)propyl functionalised silica gel In acetonitrile Knoevenagel condensation; | 99.5% |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In dichloromethane at 20℃; for 5h; Knoevenagel Condensation; | 90% |
| With piperidine In ethanol at 20℃; | |
| With sodium hydroxide In ethanol for 0.25h; air atmosphere; | |
| With piperazine immobilized by 4-benzyl spacer on silica gel In acetonitrile at 20℃; for 0.0833333h; Knoevenagel Condensation; Flow reactor; |
-
-
7311-34-4
3,5-dimethoxybenzaldehdye

-
-
105-56-6
ethyl 2-cyanoacetate

-
-
5864-90-4
3-(3,5-dimethoxyphenyl)-2-cyano acrylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With 3-(1-piperazino)propyl-functionalised silica gel In acetonitrile Knoevenagel reaction; electroosmotic flow; | 99.5% |

| Conditions | Yield |
|---|---|
| Stage #1: (4-nitrophenyl)ethanone With lithium hydroxide monohydrate In methanol at 20℃; for 1h; Stage #2: 3,5-dimethoxybenzaldehdye In methanol at 20℃; for 3h; | 99.5% |
-
-
7311-34-4
3,5-dimethoxybenzaldehdye

-
-
149-73-5
trimethyl orthoformate

-
-
59276-34-5
3,5-dimethoxybenzaldehyde dimethyl acetal

| Conditions | Yield |
|---|---|
| With amberlyst-15 In acetonitrile for 1h; electroosmos; | 99.4% |
| With amberlyst-15 In acetonitrile for 0.166667h; | 98.4% |
| With ammonium chloride In methanol for 24h; | 98% |
| With toluene-4-sulfonic acid |
-
-
7311-34-4
3,5-dimethoxybenzaldehdye

-
-
109-77-3
malononitrile

-
-
26495-19-2
β,β-dicyano-3,5-dimethoxystyrene

| Conditions | Yield |
|---|---|
| With 3-(1-piperazino)propyl functionalised silica gel In acetonitrile Knoevenagel condensation; | 99.2% |
| With 3-(1-piperazino)propyl-functionalised silica gel In acetonitrile Knoevenagel reaction; electroosmotic flow; | 99.2% |
| With piperazine In acetonitrile for 1h; Knoevenagel condensation; electroosmos; | 99.2% |
-
-
7677-24-9
trimethylsilyl cyanide

-
-
7311-34-4
3,5-dimethoxybenzaldehdye

-
-
144758-83-8
α-(3,5-dimethoxyphenyl)-α-(trimethylsilyloxy)acetonitrile

| Conditions | Yield |
|---|---|
| With C32H39Br2MgN2(1-)*C16H32LiO4(1+) In chloroform-d1 at 20℃; for 3h; Inert atmosphere; Glovebox; | 99% |
| With zinc(II) iodide In benzene for 1.5h; Ambient temperature; | 93% |
| In acetonitrile at 45℃; for 4h; | 89% |
-
-
7311-34-4
3,5-dimethoxybenzaldehdye

-
-
57367-56-3
2-tert-Butoxycarbonyl-1-methoxycarbonylethylidene(triphenyl)phosphorane

-
-
115046-50-9
2-[1-(3,5-Dimethoxy-phenyl)-meth-(E)-ylidene]-succinic acid 4-tert-butyl ester 1-methyl ester

| Conditions | Yield |
|---|---|
| In benzene for 48h; Heating; | 99% |

| Conditions | Yield |
|---|---|
| With 4H3N*4H(1+)*CuMo6O18(OH)6(4-); water; oxygen; sodium carbonate at 50℃; under 760.051 Torr; for 12h; | 99% |
| With C4H11FeMo6NO24(3-)*3C16H36N(1+); water; oxygen; sodium carbonate at 50℃; under 760.051 Torr; for 8h; Green chemistry; | 95% |
| With sodium perborate In acetic acid at 45 - 50℃; for 8h; | 83% |
-
-
7311-34-4
3,5-dimethoxybenzaldehdye

-
-
19179-31-8
3,5-dimethoxy-benzonitrile

| Conditions | Yield |
|---|---|
| With oxygen; copper; ammonium chloride In pyridine at 60℃; for 24h; | 99% |
| With ammonium sulfate; sodium carbonate; sulfur In dimethyl sulfoxide at 120℃; for 10h; Sealed tube; | 99% |
| With trimethylsilylazide; zirconium(IV) chloride In acetonitrile at 20℃; for 0.333333h; | 69% |
| With ammonia; iodine In tetrahydrofuran; water at 20℃; |
-
-
7311-34-4
3,5-dimethoxybenzaldehdye

-
-
2065-66-9
methyl-triphenylphosphonium iodide

-
-
40243-87-6
3,5-dimethoxystyrene

| Conditions | Yield |
|---|---|
| Stage #1: methyl-triphenylphosphonium iodide With sodium hydride In tetrahydrofuran; mineral oil at 0℃; for 1h; Wittig Olefination; Stage #2: 3,5-dimethoxybenzaldehdye In tetrahydrofuran; mineral oil at 0 - 20℃; for 10h; Wittig Olefination; | 99% |
| Stage #1: methyl-triphenylphosphonium iodide With potassium tert-butylate In diethyl ether at 20℃; for 2h; Wittig reaction; Stage #2: 3,5-dimethoxybenzaldehdye In diethyl ether at 0 - 20℃; Wittig reaction; | 96% |
| With potassium tert-butylate In N,N-dimethyl-formamide at -75℃; for 4h; Inert atmosphere; | 86% |
-
-
7311-34-4
3,5-dimethoxybenzaldehdye

-
-
1779-49-3
Methyltriphenylphosphonium bromide

-
-
40243-87-6
3,5-dimethoxystyrene

| Conditions | Yield |
|---|---|
| Stage #1: Methyltriphenylphosphonium bromide With potassium tert-butylate In tetrahydrofuran at 20℃; for 0.5h; Inert atmosphere; Stage #2: 3,5-dimethoxybenzaldehdye In tetrahydrofuran at -78 - 20℃; Inert atmosphere; | 99% |
| Stage #1: Methyltriphenylphosphonium bromide With sodium hydride In tetrahydrofuran; mineral oil for 1h; Wittig Olefination; Inert atmosphere; Reflux; Stage #2: 3,5-dimethoxybenzaldehdye In tetrahydrofuran; mineral oil for 2h; Wittig Olefination; Inert atmosphere; Reflux; | 99% |
| With potassium tert-butylate In tetrahydrofuran at -70 - 20℃; for 2h; Wittig reaction; | 96% |
3,5-Dimethoxybenzaldehyde Chemical Properties
Molecule structure of 3,5-Dimethoxybenzaldehyde (CAS NO.7311-34-4):
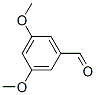
IUPAC Name: 3,5-Dimethoxybenzaldehyde
Molecular Weight: 166.1739 g/mol
Molecular Formula: C9H10O3
Density: 1.114 g/cm3
Melting Point: 45-48 °C(lit.)
Boiling Point: 276.5 °C at 760 mmHg
Flash Point: 119 °C
Index of Refraction: 1.534
Molar Refractivity: 46.36 cm3
Molar Volume: 149.1 cm3
Surface Tension: 36.1 dyne/cm
Enthalpy of Vaporization: 51.5 kJ/mol
Vapour Pressure: 0.00478 mmHg at 25 °C
Water Solubility: insoluble
Sensitive: air sensitive
XLogP3-AA: 1.3
H-Bond Acceptor: 3
Rotatable Bond Count: 3
Exact Mass: 166.062994
MonoIsotopic Mass: 166.062994
Topological Polar Surface Area: 35.5
Heavy Atom Count: 12
Canonical SMILES: COC1=CC(=CC(=C1)C=O)OC
InChI: InChI=1S/C9H10O3/c1-11-8-3-7(6-10)4-9(5-8)12-2/h3-6H,1-2H3
InChIKey: VFZRZRDOXPRTSC-UHFFFAOYSA-N
EINECS: 230-772-6
Product Categories: Carbonyl Compounds;Aromatic Aldehydes & Derivatives (substituted); Adehydes, Acetals & Ketones; Anisoles, Alkyloxy Compounds & Phenylacetates; Building Blocks for Dendrimers; Functional Materials; Aldehydes; C9; Carbonyl Compounds
3,5-Dimethoxybenzaldehyde Uses
3,5-Dimethoxybenzaldehyde (CAS NO.7311-34-4) is mainly used for pharmaceutical intermediates.
3,5-Dimethoxybenzaldehyde Safety Profile
Hazard Codes:  Xi
Xi
Risk Statements: 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 26-36-24/25
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36:Wear suitable protective clothing.
S24/25:Avoid contact with skin and eyes.
WGK Germany: 3
F: 9
Hazard Note: Irritant
3,5-Dimethoxybenzaldehyde Specification
3,5-Dimethoxybenzaldehyde (CAS NO.7311-34-4) is also named as Benzaldehyde, 3,5-dimiethoxy ; 3,5-Dimethoxybenzaldehyde 98% . 3,5-Dimethoxybenzaldehyde (CAS NO.7311-34-4) is white to beige crystalline solid.
Related Products
- 3,10-Diaminotricyclo(5.2.1.0(sup 2,6))decane
- 3,10-Dinitrophenanthrene
- 3-((10-ETHYL-11-(p-HYDROXYPHENYL)DIBENZ-(B,F)OXEPIN-3-YL)OXY)-1,2-PROPANEDIOL HYDRATE (4:1)
- 3-(1,1,2,2-Tetrafluoroethoxy)aniline
- 3-(1,1,2,2-Tetrafluoroethoxy)benzaldehyde
- 3-(1,1,2,2-Tetrafluoroethoxy)bromobenzene
- 3-(1,1,2,2-Tetrafluoroethoxy)toluene
- 3-[1,1'-Biphenyl]-4-yl-1,2,3,4-tetrahydro-1-naphthol
- 3,11-Dichloro-6,11-dihydro-6-methyldibenzo[c,f][1,2]thiazepine 5,5-dioxide
- 3-[(1,1-Dimethyl-2-hydroxyethyl)amino]-2-hydroxypropanesulfonicacid
- 7311-63-9
- 7311-64-0
- 7311-87-7
- 73119-06-9
- 7311-93-5
- 73120-52-2
- 7312-10-9
- 73121-56-9
- 7312-24-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View