-
Name
3-Aminophenol
- EINECS 209-711-2
- CAS No. 591-27-5
- Article Data214
- CAS DataBase
- Density 1.2±0.1 g/cm3
- Solubility Soluble in hot water, ethanol, ethyl ether and pentanol, slightly soluble in water and benzene
- Melting Point 121 °C
- Formula C6H7NO
- Boiling Point 298.566 °C at 760 mmHg
- Molecular Weight 109.128
- Flash Point 116.4±19.8 °C
- Transport Information UN 2512 6.1/PG 3
- Appearance White crystals or off-white flakes
- Safety 28-61
- Risk Codes 20/22-51/53
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, N
N
- Synonyms Phenol,m-amino- (8CI);(3-Hydroxyphenyl)amine;1-Amino-3-hydroxybenzene;3-Hydroxybenzenamine;C.I. OxidationBase 7;Fourrine 65;Fourrine EG;Nako TEG;m-Hydroxyphenylamine;
- PSA 46.25000
- LogP 1.55560
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogen In ethanol at 60℃; under 7500.75 Torr; for 2h; | 100% |
| With palladium on activated charcoal; hydrogen In methanol at 20℃; for 12h; | 100% |
| With sodium tetrahydroborate In methanol; water at 20℃; for 3h; | 100% |
-
-
147985-36-2
m-tetrahydropyran-2-yloxyaminobenzene

-
-
591-27-5
m-Hydroxyaniline

| Conditions | Yield |
|---|---|
| With β‐cyclodextrin In water at 60℃; for 0.133333h; Microwave irradiation; | 99% |
| With ytterbium(III) chloride In neat (no solvent) at 70 - 120℃; for 0.0333333h; Microwave irradiation; Green chemistry; | 95% |

| Conditions | Yield |
|---|---|
| With pyrrolidine; hydrogen; 5% rhodium-on-charcoal; iron(II) acetate In tetrahydrofuran at 20℃; for 2.5h; | A 98% B 0.5% |
| With hydrogen; 5% rhodium-on-charcoal; nickel(II) nitrate In tetrahydrofuran; water at 20℃; for 15h; | A 92% B 2% |
| With hydrogen; 5% rhodium-on-charcoal; iron(II) acetate In tetrahydrofuran at 20℃; for 33h; | A 92% B 0.8% |
-
-
96649-05-7
3-(methoxymethoxy)aniline

-
-
591-27-5
m-Hydroxyaniline

| Conditions | Yield |
|---|---|
| With β‐cyclodextrin In water at 60℃; for 0.15h; Microwave irradiation; | 98% |
| With ytterbium(III) chloride In neat (no solvent) at 70 - 120℃; for 0.0333333h; Microwave irradiation; Green chemistry; | 93% |
-
-
51642-25-2
3-azidophenol

-
-
591-27-5
m-Hydroxyaniline

| Conditions | Yield |
|---|---|
| With ammonium hydroxide at 90℃; for 1h; | 97% |
| With aluminium(III) iodide In benzene for 0.25h; Reduction; Heating; | 92% |
| With triphenylphosphine-2-carboxamide In tetrahydrofuran; water at 20℃; for 2h; Staudinger Azide Reduction; | 90% |
-
-
100367-37-1
3-amino-4-bromophenol

-
-
591-27-5
m-Hydroxyaniline

| Conditions | Yield |
|---|---|
| With sodium sulfite In water at 60℃; for 18h; Green chemistry; | 96% |
-
-
19962-06-2
tert-butyl 3-hydroxyphenylcarbamate

-
-
591-27-5
m-Hydroxyaniline

| Conditions | Yield |
|---|---|
| With water at 100℃; for 1h; | 95% |

| Conditions | Yield |
|---|---|
| With [Cu2(2,7-bis(pyridin-2-yl)-l,8-naphthyridine)(OH)(CF3COO)3]; tetrabutylammomium bromide; ammonia; caesium carbonate In water at 110 - 120℃; for 16h; Sealed tube; | 95% |

| Conditions | Yield |
|---|---|
| With sulfuric acid; sodium nitrite at 10 - 90℃; | 92.8% |
| Diazotization.ueber mehrere Stufen; |
-
-
121942-75-4
3-[(tert-butyldimethylsilyl)oxy]aniline

-
-
591-27-5
m-Hydroxyaniline

| Conditions | Yield |
|---|---|
| With sodium phosphate dodecahydrate In N,N-dimethyl-formamide at 20℃; for 2h; | 92% |
| With potassium hydroxide In ethanol at 20℃; for 2h; | 91% |
-
-
24318-00-1
3-(benzyloxy)nitrobenzol

-
-
591-27-5
m-Hydroxyaniline

| Conditions | Yield |
|---|---|
| With 5%-palladium/activated carbon; hydrogen In ethanol at 60℃; under 3000.3 Torr; for 3h; | 92% |
-
-
100367-36-0
2-bromo-3-hydroxyaniline

-
-
591-27-5
m-Hydroxyaniline

| Conditions | Yield |
|---|---|
| With sodium sulfite In water at 60℃; for 18h; Green chemistry; | 91% |
-
-
30418-59-8
3-Aminophenylboronic acid

-
-
591-27-5
m-Hydroxyaniline

| Conditions | Yield |
|---|---|
| With 1,10-Phenanthroline; copper(II) sulfate; potassium hydroxide In water at 20℃; for 3h; | 90% |
| With water; caesium carbonate; hydrazine hydrate at 80℃; for 12h; | 85% |
| With oxygen; N-ethyl-N,N-diisopropylamine In acetone at 25℃; under 760.051 Torr; for 65h; Catalytic behavior; Irradiation; | 32 %Chromat. |

| Conditions | Yield |
|---|---|
| In water; N,N-dimethyl-formamide at 20℃; for 3h; | 88% |
-
-
51581-40-9
3-pyridyl diethylcarbamate

-
-
591-27-5
m-Hydroxyaniline

| Conditions | Yield |
|---|---|
| With zirconocene dichloride In tetrahydrofuran at 20℃; Inert atmosphere; | 87% |

| Conditions | Yield |
|---|---|
| With 10% Pd/C; cyclohexa-1,4-diene In ethyl acetate at 100℃; for 0.5h; Microwave irradiation; | 86% |
| In ethanol for 2h; Cooling with ice; | 48% |
| Multi-step reaction with 2 steps 1: 94 percent 2: 71.4 percent / TFSA / trifluoroacetic acid / 0 °C View Scheme |

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid In trifluoroacetic acid at 0℃; | A 28.5% B 71.4% |

| Conditions | Yield |
|---|---|
| With mercury(II) diacetate In acetonitrile for 20h; Heating; | 56% |

| Conditions | Yield |
|---|---|
| With 5%-palladium/activated carbon; hydrazine hydrate; lithium hydroxide In 1,4-dioxane at 170℃; for 16h; Molecular sieve; Inert atmosphere; | 44% |
| With ammonium hydroxide; ammonium chloride at 200℃; | |
| With ammonium chloride; ammonia at 200℃; |
-
-
30418-59-8
3-Aminophenylboronic acid

-
A
-
2243-47-2
3-biphenyl amine

-
B
-
2050-89-7
3,3'-diaminobiphenyl

-
C
-
591-27-5
m-Hydroxyaniline

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0) In toluene for 20h; Dimerization; Heating; | A 5% B 44% C 14% |

-
-
51985-06-9
3-hydroxy-alpha-methylstyrene

-
-
591-27-5
m-Hydroxyaniline

| Conditions | Yield |
|---|---|
| Stage #1: 3-hydroxy-alpha-methylstyrene With sodium azide; sulfuric acid In hexane; water at 20 - 40℃; for 4h; Stage #2: With sodium hydroxide In water at 0℃; for 0.333333h; Reagent/catalyst; | 40% |

| Conditions | Yield |
|---|---|
| With hydrogen fluoride; dihydrogen peroxide; antimony pentafluoride at -20℃; for 0.25h; | A 14% B 20.5% C 36% |
| With dihydrogen peroxide; antimony pentafluoride In hydrogen fluoride at -20℃; for 0.25h; | A 14% B 20.5% C 36% |
| With dihydrogen peroxide; antimony pentafluoride In hydrogen fluoride at -20℃; for 0.25h; Mechanism; Product distribution; | A 14% B 20.5% C 36% |
| With bismuth(III) oxobromide; 18O-labeled water; oxygen-18 Reagent/catalyst; Photolysis; |


| Conditions | Yield |
|---|---|
| mit den ueblichen Reduktionsmitteln; | |
| bei Einw. der ueblichen Reduktionsmittel; |

| Conditions | Yield |
|---|---|
| bei der Destillation; |


| Conditions | Yield |
|---|---|
| With sodium hydroxide; water at 280 - 290℃; | |
| With sodium hydroxide at 280 - 290℃; | |
| With sodium hydroxide; water at 280 - 290℃; |

| Conditions | Yield |
|---|---|
| With aluminum oxide; ammonia at 420℃; |
-
-
938-10-3
phenylsulfonyl azide

-
-
108-95-2
phenol

-
A
-
123-30-8
4-amino-phenol

-
B
-
95-55-6
2-amino-phenol

-
C
-
591-27-5
m-Hydroxyaniline

| Conditions | Yield |
|---|---|
| at 120℃; Product distribution; anschl. Erwaermen mit wss. HCl; |


| Conditions | Yield |
|---|---|
| In water at 25℃; Product distribution; Mechanism; electrochemical reduction in buffered aqueous solution; pH dependence; | |
| In ethanol; water at 25℃; Rate constant; Mechanism; Product distribution; polarographic reduction; potential-dependent rate constants kf,h, αna; pH = 1.81-11.00; |
-
-
10603-61-9
m-hydroxyphenyl-hydroxylamine

-
-
591-27-5
m-Hydroxyaniline

| Conditions | Yield |
|---|---|
| With Britton-Robbinson buffer; N-dodecylpyridinium chloride In ethanol at 25℃; Kinetics; Mechanism; electric current; | |
| With Britton-Robbinson buffer; sodium dodecyl-sulfate In ethanol at 25℃; Kinetics; Mechanism; electric current; | |
| With Britton-Robbinson buffer; Triton X-100 In ethanol at 25℃; Kinetics; Mechanism; electric current; |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 2h; | 100% |
| In tetrahydrofuran at 20℃; for 2h; | 100% |
| With triethylamine In diethyl ether at 20℃; for 18h; | 99% |

| Conditions | Yield |
|---|---|
| With zirconium(IV) phosphate at 110℃; for 0.166667h; Pechmann condensation; Microwave irradiation; chemoselective reaction; | 100% |
| With silica gel supported zirconyl chloride octahydrate at 90℃; for 0.583333h; Pechmann condensation reaction; | 98% |
| With tetrakis(actonitrile)copper(I) hexafluorophosphate at 25℃; for 0.166667h; Pechmann condensation; neat (no solvent); | 98% |
-
-
1120-71-4
1,3-propanesultone

-
-
591-27-5
m-Hydroxyaniline

-
-
52962-41-1
3-((3-hydroxyphenyl)amino)propane-1-sulfonic acid

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; | 100% |
| In butan-1-ol for 0.5h; Heating; | 80% |
| for 0.5h; Reflux; | 80% |
-
-
999-97-3
1,1,1,3,3,3-hexamethyl-disilazane

-
-
591-27-5
m-Hydroxyaniline

-
-
36309-43-0
3-((trimethylsilyl)oxy)aniline

| Conditions | Yield |
|---|---|
| phosphotungstic acid at 55 - 60℃; for 0.75h; | 100% |
| With phosphotungstic acid at 55 - 60℃; for 0.75h; | 99% |
| With silica triflate at 20℃; for 0.0333333h; | 90% |
-
-
120-14-9
3,4-dimethoxy-benzaldehyde

-
-
591-27-5
m-Hydroxyaniline

-
-
23837-30-1
3,4-Methoxybenzyliden-m-hydroxyanilin

| Conditions | Yield |
|---|---|
| In toluene Heating; | 100% |
-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
591-27-5
m-Hydroxyaniline

-
-
121942-75-4
3-[(tert-butyldimethylsilyl)oxy]aniline

| Conditions | Yield |
|---|---|
| With 1H-imidazole In tetrahydrofuran at 20℃; Inert atmosphere; | 100% |
| With 1H-imidazole In dichloromethane at 20℃; for 15h; | 99% |
| With dmap; triethylamine In dichloromethane at 20℃; for 6h; | 99.93% |
-
-
501-53-1
benzyl chloroformate

-
-
591-27-5
m-Hydroxyaniline

-
-
19972-88-4
3-Hydroxycarbanilic Acid Benzyl Ester

| Conditions | Yield |
|---|---|
| In diethyl ether for 18h; Ambient temperature; | 100% |
| With sodium hydrogencarbonate In 1,4-dioxane; water at 0 - 20℃; for 13h; | 68% |
| With triethylamine In diethyl ether at 20℃; for 18h; | 51% |
| With sodium carbonate In tetrahydrofuran; water at 0 - 20℃; for 1h; |
-
-
16801-19-7
2-[2-(vinyloxy)ethoxymethyl]oxirane

-
-
591-27-5
m-Hydroxyaniline

| Conditions | Yield |
|---|---|
| at 40 - 50℃; for 1h; | 100% |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
591-27-5
m-Hydroxyaniline

-
-
19962-06-2
tert-butyl 3-hydroxyphenylcarbamate

| Conditions | Yield |
|---|---|
| In tetrahydrofuran Reflux; | 100% |
| With triethylamine | 99% |
| In tetrahydrofuran for 24h; Heating; | 96% |
-
-
107866-54-6
1-acetyl-1H-1,2,3-triazolo<4,5-b>pyridine

-
-
591-27-5
m-Hydroxyaniline

-
-
621-42-1
meta-hydroxyacetanilide

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 1h; Ambient temperature; | 100% |
-
-
591-27-5
m-Hydroxyaniline

| Conditions | Yield |
|---|---|
| With d(4)-methanol; triethylamine for 336h; Ambient temperature; | 100% |
-
-
51640-36-9
2-chloro-1,3-thiazole-5-carbonitrile

-
-
591-27-5
m-Hydroxyaniline

-
-
946885-69-4
2-(3-aminophenoxy)-1,3-thiazole-5-carbonitrile

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; | 100% |
-
-
591-27-5
m-Hydroxyaniline

-
-
13790-39-1
6,7-dimethoxy-4-chloroquinazoline

-
-
1188908-37-3
3-(6,7-dimethoxyquinazolin-4-yloxy)-benzenamine

| Conditions | Yield |
|---|---|
| Stage #1: m-Hydroxyaniline With caesium carbonate In tetrahydrofuran at 20℃; for 0.5h; Stage #2: 6,7-dimethoxy-4-chloroquinazoline In tetrahydrofuran at 50℃; for 24h; | 100% |
| With tetrabutylammomium bromide; sodium hydroxide In butanone for 0.5h; Reflux; | 93% |
| With tetrabutylammomium bromide; sodium hydroxide In water; butanone Reflux; | 93% |
| Stage #1: m-Hydroxyaniline With potassium tert-butylate In N,N-dimethyl-formamide at 20℃; for 1h; Inert atmosphere; Stage #2: 6,7-dimethoxy-4-chloroquinazoline With potassium carbonate In N,N-dimethyl-formamide at 80 - 85℃; for 2h; | 77.9% |
-
-
1583284-78-9
4-chloro-6-(methylthio)-1-(prop-2-ynyl)-1H-pyrazolo[3,4-d]pyrimidine

-
-
591-27-5
m-Hydroxyaniline

-
-
1583284-89-2
3-(6-(methylthio)-1-(prop-2-ynyl)-1H-pyrazolo[3,4-d]pyrimidin-4-ylamino)phenol

| Conditions | Yield |
|---|---|
| In ethanol for 12h; Reflux; Inert atmosphere; | 100% |
-
-
1583284-79-0
1-((1-benzyl-1H-1,2,3-triazol-4-yl)methyl)-4-chloro-6-(methylthio)-1H-pyrazolo[3,4-d]pyrimidine

-
-
591-27-5
m-Hydroxyaniline

-
-
1583285-03-3
3-(1-((1-benzyl-1H-1,2,3-triazol-4-yl)methyl)-6-(methylthio)-1H-pyrazolo[3,4-d]pyrimidin-4-ylamino)phenol

| Conditions | Yield |
|---|---|
| In ethanol for 12h; Reflux; Inert atmosphere; | 100% |
-
-
1253889-39-2
3-(3,5-bis(trifluoromethyl)phenylimino)isobenzofuran-1(3H)-one

-
-
591-27-5
m-Hydroxyaniline

-
-
1615737-65-9
N1-(3,5-bis(trifluoromethyl)phenyl)-N2-(3-hydroxyphenyl)phthalamide

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; Inert atmosphere; | 100% |

-
-
591-27-5
m-Hydroxyaniline

| Conditions | Yield |
|---|---|
| With caesium carbonate In dimethyl sulfoxide at 120℃; for 1h; Sealed tube; | 100% |

-
-
591-27-5
m-Hydroxyaniline

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 12h; | 99.16% |
-
-
541-41-3
chloroformic acid ethyl ester

-
-
591-27-5
m-Hydroxyaniline

-
-
7159-96-8
ethyl N-(3-hydroxyphenyl)carbamate

| Conditions | Yield |
|---|---|
| In ethyl acetate Heating; | 99% |
| In ethyl acetate for 1h; Reflux; | 93% |
| In ethyl acetate for 0.5h; Reflux; | 93% |
-
-
292638-85-8
acrylic acid methyl ester

-
-
591-27-5
m-Hydroxyaniline

-
-
59486-18-9
3-[(3-hydroxyphenyl)(2-methoxycarbonylethyl)amino]propionic acid methyl ester

| Conditions | Yield |
|---|---|
| With acetic acid at 80℃; for 120h; | 99% |
| With sodium bromide In acetic acid at 95℃; for 19h; Inert atmosphere; | 86% |
| With acetic acid; sodium bromide at 95℃; for 19h; Inert atmosphere; | 78.4% |
| With acetic acid; copper(l) chloride for 20h; Reflux; | 65% |

| Conditions | Yield |
|---|---|
| With pyridine hydrogenfluoride; sodium nitrite 1.) O deg C, 20 min, 2.) 60 deg C, 1 h; | 99% |
| Stage #1: m-Hydroxyaniline With hydrogen fluoride; sodium nitrite at -10 - -5℃; for 1h; Inert atmosphere; Stage #2: at 48 - 54℃; Concentration; Time; Temperature; | 45.6% |
| With hydrogen fluoride; sodium nitrite at 0℃; |

| Conditions | Yield |
|---|---|
| With triethylamine In chloroform at 60℃; for 3h; | 99% |
-
-
122664-30-6
N-acetyl-β-phenylacetoacetamide

-
-
591-27-5
m-Hydroxyaniline

-
-
122664-42-0
2,6-dimethyl-5-phenyl-1-(3'-hydroxyphenyl)-4-oxo-1,4-dihydropyrimidine

| Conditions | Yield |
|---|---|
| In acetic acid for 1h; | 99% |
| In acetic acid for 1h; Heating; | 99% |
| With acetic acid for 1h; Heating; | 60% |
-
-
87-13-8
diethyl 2-ethoxymethylenemalonate

-
-
591-27-5
m-Hydroxyaniline

-
-
84165-81-1
1,3-diethyl 2-(((3-hydroxyphenyl)amino)methylidene)propanedioate

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 0.25h; | 99% |
| With zinc(II) chloride In ethanol Heating; | 89% |

| Conditions | Yield |
|---|---|
| With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; | 99% |
| With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; | 99% |
-
-
86-98-6
4,7-dichloroquinoline

-
-
591-27-5
m-Hydroxyaniline

-
-
154179-33-6
4-(3'-hydroxyphenyl)amino-7-chloroquinoline

| Conditions | Yield |
|---|---|
| In ethanol Reflux; | 99% |
| for 0.25h; microwave irradiation; | 75% |
| With hydrogenchloride; potassium iodide In ethanol Heating; |

| Conditions | Yield |
|---|---|
| With dimanganese decacarbonyl at 180℃; for 1h; | 99% |
-
-
135636-66-7
butane-2,3-dione mono-oxime

-
-
591-27-5
m-Hydroxyaniline

| Conditions | Yield |
|---|---|
| In ethanol Reflux; | 99% |
| In ethanol Reflux; | 99% |
3-Aminophenol Specification
3-Aminophenol is an organic compound with the formula C6H7NO, and its systematic name is the same with the product name. With the CAS registry number 591-27-5, it is also named as 3-Amino-1-hydroxybenzene. It belongs to the product categories of Aromatic Phenols; Anilines, Aromatic Amines and Nitro Compounds; Organics; Phenoles and thiophenoles; API intermediates; Organic Chemicals; Organic Building Blocks; Oxygen Compounds; Phenols; AM to AQ Chemical Class; Alpha sort; Amines; Aromatics; Pesticides & Metabolites. Its EINECS number is 209-711-2. In addition, the molecular weight is 109.13. Its classification codes are: (1)Mutation data; (2)Reproductive Effect; (3)Skin / Eye Irritant. This product should be sealed and kept in dark place. Moreover, it should be protected from heat and moisture. It is an aromatic amine and aromatic alcohol. It is an isomer of 2-aminophenol and 4-aminophenol. This chemical is used in organic synthesis. It is an intermediate of dyes and p-aminosalicylic acid. It is also used in the production of antioxidants, stabilizer, developers and color film.
Physical properties of 3-Aminophenol are: (1)ACD/LogP: 0.34±0.21; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0.32; (4)ACD/LogD (pH 7.4): 0.34; (5)ACD/BCF (pH 5.5): 1.01; (6)ACD/BCF (pH 7.4): 1.06; (7)ACD/KOC (pH 5.5): 34.64; (8)ACD/KOC (pH 7.4): 36.34; (9)#H bond acceptors: 2; (10)#H bond donors: 3; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 46.25 Å2; (13)Index of Refraction: 1.637; (14)Molar Refractivity: 32.4±0.3 cm3; (15)Molar Volume: 90.1±3.0 cm3; (16)Polarizability: 12.8±0.5×10-24cm3; (17)Surface Tension: 57.4±3.0 dyne/cm; (18)Density: 1.2±0.1 g/cm3; (19)Flash Point: 116.4±19.8 °C; (20)Enthalpy of Vaporization: 56.0±3.0 kJ/mol; (21)Boiling Point: 298.566 °C at 760 mmHg; (22)Vapour Pressure: 0.0±0.6 mmHg at 25°C.
Preparation: this chemical can be prepared by 3-nitro-phenol at the ambient temperature. This reaction will need reagent NaBH4-SbCl3 and solvent ethanol with the reaction time of 10 min. The yield is about 93%.
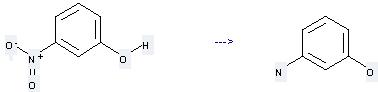
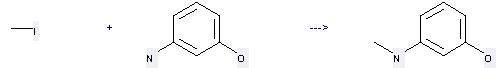
Uses of 3-Aminophenol: it can be used to produce 3-Methylamino-phenol at the temperature of 100 °C. This reaction will need reagent potassium carbonate and solvent dimethylformamide with the reaction time of 2 hours. The yield is about 65%.
When you are using this chemical, please be cautious about it as the following:
This chemical is harmful by inhalation and if swallowed. It is toxic to aquatic organisms as it may cause long-term adverse effects in the aquatic environment. After contact with skin, you should wash immediately with plenty of ... (to be specified by the manufacturer). You must avoid releasing it to the environment, and you need to refer to special instructions/safety data sheet.
You can still convert the following datas into molecular structure:
(1)SMILES: c1cc(cc(c1)O)N
(2)Std. InChI: InChI=1S/C6H7NO/c7-5-2-1-3-6(8)4-5/h1-4,8H,7H2
(3)Std. InChIKey: CWLKGDAVCFYWJK-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| bird - wild | LD50 | oral | 237mg/kg (237mg/kg) | Archives of Environmental Contamination and Toxicology. Vol. 12, Pg. 355, 1983. | |
| mammal (species unspecified) | LD50 | unreported | 1245mg/kg (1245mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 48(9), Pg. 19, 1983. | |
| mouse | LD50 | intraperitoneal | 150mg/kg (150mg/kg) | National Technical Information Service. Vol. AD691-490, | |
| mouse | LD50 | oral | 401mg/kg (401mg/kg) | BEHAVIORAL: EXCITEMENT PERIPHERAL NERVE AND SENSATION: SPASTIC PARALYSIS WITH OR WITHOUT SENSORY CHANGE LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 32(1), Pg. 49, 1988. |
| mouse | LD50 | unreported | 225mg/kg (225mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 50(3), Pg. 4, 1985. | |
| quail | LD50 | oral | 750mg/kg (750mg/kg) | Archives of Environmental Contamination and Toxicology. Vol. 12, Pg. 355, 1983. | |
| rat | LC50 | inhalation | 1162mg/m3 (1162mg/m3) | BEHAVIORAL: EXCITEMENT PERIPHERAL NERVE AND SENSATION: SPASTIC PARALYSIS WITH OR WITHOUT SENSORY CHANGE LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 32(1), Pg. 49, 1988. |
| rat | LD50 | oral | 924mg/kg (924mg/kg) | LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES PERIPHERAL NERVE AND SENSATION: SPASTIC PARALYSIS WITH OR WITHOUT SENSORY CHANGE BEHAVIORAL: EXCITEMENT | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 32(1), Pg. 49, 1988. |
| rat | LD50 | unreported | 1125mg/kg (1125mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 50(3), Pg. 4, 1985. | |
| rat | LDLo | intraperitoneal | 1gm/kg (1000mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 131, Pg. 151, 1961. |
Related Products
- 3-Aminophenol
- 3-Aminophenol hemisulfate
- 3-Aminophenol hydrochloride
- 59128-09-5
- 591-28-6
- 59128-90-4
- 59128-97-1
- 59129-18-9
- 59129-52-1
- 59129-79-2
- 59130-69-7
- 591-31-1
- 5913-13-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View