-
Name
3-Cyanopyridine
- EINECS 202-863-0
- CAS No. 100-54-9
- Article Data362
- CAS DataBase
- Density 1.159 g/cm3
- Solubility 140 g/L (20 °C) in water
- Melting Point 48-52 °C(lit.)
- Formula C6H4N2
- Boiling Point 202.985 °C at 760 mmHg
- Molecular Weight 104.111
- Flash Point 84.444 °C
- Transport Information
- Appearance white to beige solid
- Safety 22-24/25-36/37/39-26
- Risk Codes 22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Nicotinonitrile(8CI);3-Azabenzonitrile;3-Pyridinenitrile;3-Pyridylcyanide;3-Pyridylcarbonitrile;NSC 17558;Nicotinic acid nitrile;b-Cyanopyridine;3-Pyridinecarbonitrile;
- PSA 36.68000
- LogP 0.95328
Synthetic route

| Conditions | Yield |
|---|---|
| With N-methyl-N-trimethylsilyl-2,2,2-trifluoroacetamide; copper(l) chloride In toluene at 100℃; for 24h; | 99% |
| With 3 A molecular sieve at 150 - 500℃; under 0.001 Torr; for 0.666667h; Pyrolysis; | 98% |
| With oxalyl dichloride; triethylamine; Triphenylphosphine oxide In acetonitrile at 20℃; for 0.166667h; | 96% |

| Conditions | Yield |
|---|---|
| With ammonium sulfate; sodium carbonate; sulfur In dimethyl sulfoxide at 120℃; for 10h; Sealed tube; | 99% |
| With ammonia; oxygen In ethanol; water for 1.5h; Reflux; | 94% |
| With hydroxylamine hydrochloride; pyrographite; methanesulfonyl chloride at 100℃; for 1h; | 92% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-hydroxymethylpyridin With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; tert-butylhypochlorite In dichloromethane at 20℃; for 1h; Inert atmosphere; Stage #2: With ammonia; iodine In dichloromethane; water at 20℃; for 2h; Inert atmosphere; | 99% |
| With ammonia; oxygen In tert-Amyl alcohol; water at 100℃; under 3750.38 Torr; for 4h; Autoclave; High pressure; | 99% |
| With potassium phosphate; ammonium formate In acetonitrile at 115℃; for 16h; Sealed tube; Green chemistry; | 91% |

| Conditions | Yield |
|---|---|
| With C18H14CuIN4 In acetonitrile at 20℃; for 24h; Inert atmosphere; Sealed tube; UV-irradiation; | 99% |

| Conditions | Yield |
|---|---|
| With manganese; ammonia; oxygen; pyrographite at 335℃; Temperature; | 98.7% |
| With ammonia In water at 200 - 300℃; Concentration; | 97% |
| With aluminum oxide; ammonia; oxygen at 300℃; Concentration; Temperature; | 93.1% |

| Conditions | Yield |
|---|---|
| With Palladium Nanoparticles with two shape-persistent covalent cages CC1' In N,N-dimethyl-formamide at 140℃; for 15h; Reagent/catalyst; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| With tellurium tetrachloride; triethylamine In chloroform for 3h; Ambient temperature; | 96% |
| With methylene blue; 1,8-diazabicyclo[5.4.0]undec-7-ene In acetonitrile at 25℃; Sealed tube; Irradiation; | 95% |
| With bis(tri-n-butyltin)oxide In benzene for 0.5h; Heating; | 92% |

| Conditions | Yield |
|---|---|
| With thionyl chloride; polyvinylpyrrolidone In dichloromethane at 20℃; for 0.25h; Dehydration; | 95% |
| With 1,4-diaza-bicyclo[2.2.2]octane; trichlorophosphate In dichloromethane at 20℃; for 0.0833333h; | 94% |
| With aluminium trichloride; sodium iodide In acetonitrile for 2.5h; Heating; | 92% |

| Conditions | Yield |
|---|---|
| With tributyltin chloride; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene; tris(dibenzylideneacetone)dipalladium (0) In acetonitrile at 22℃; for 3h; | 95% |

| Conditions | Yield |
|---|---|
| With sodium carbonate; palladium diacetate In 1-methyl-pyrrolidin-2-one; Hexadecane at 160℃; for 16h; | 95% |
| With sodium carbonate; cyclopalladated ferrocenylimine tricyclohexylphosphine In 1-methyl-pyrrolidin-2-one at 140℃; for 18h; | 82% |
| With sodium carbonate In N,N-dimethyl-formamide at 120℃; for 16h; | 57% |
| With tetra(adamantyl)biphosphine; palladium diacetate; sodium carbonate In 1-methyl-pyrrolidin-2-one at 140℃; for 16h; Inert atmosphere; | 66 %Chromat. |

| Conditions | Yield |
|---|---|
| With 1,1'-bis-(diphenylphosphino)ferrocene; [1,1'-bis(diphenylphosphino)ferrocene]nickel(II) chloride; zinc In N,N-dimethyl-formamide at 80℃; for 3h; Reagent/catalyst; Concentration; Temperature; Solvent; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| With sodium carbonate; potassium ferrocyanide In N,N-dimethyl-formamide at 120℃; for 5h; | 94% |

| Conditions | Yield |
|---|---|
| With tributyltin chloride; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene; tris(dibenzylideneacetone)dipalladium (0) In acetonitrile at 80℃; for 17h; | 93% |
| With dibromobis(triphenylphosphine)nickel(II); triphenylphosphine; zinc In N,N,N,N,N,N-hexamethylphosphoric triamide at 60℃; for 16h; | 88% |
| With dibromobis(triphenylphosphine)nickel(II); triphenylphosphine; zinc In N,N,N,N,N,N-hexamethylphosphoric triamide at 60℃; for 16h; Product distribution; other heteroaromatic halides, var. solv., temp., also NaCN; selective reactivity of var. heteroaromatic halides; | |
| dibromobis(triphenylphosphine)nickel(II) In acetonitrile at 60℃; for 8h; |

| Conditions | Yield |
|---|---|
| With aluminum oxide In N,N-dimethyl-formamide at 120℃; for 12h; Inert atmosphere; | 93% |
| With 1-methyl-1H-imidazole; oxygen; copper(ll) bromide In dimethyl sulfoxide at 100℃; for 24h; | 92% |
| With potassium hydroxide; nickel copper formate; (Bu4N)2S2O8 In dichloromethane at 20℃; for 12h; Oxidation; | 90% |
-
-
65943-95-5
2-(pyridin-3-yl)-1,3,4-oxadiazole

-
-
100-54-9
pyridine-3-carbonitrile

| Conditions | Yield |
|---|---|
| With sodium t-butanolate In N,N-dimethyl-formamide at 20℃; for 8h; | 93% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 120℃; for 12h; | 93% |
| With potassium carbonate In N,N-dimethyl-formamide at 120℃; for 15h; Schlenk technique; Green chemistry; | 90% |
| With sodium carbonate In N,N-dimethyl-formamide at 120℃; for 2h; Catalytic behavior; | 90% |
-
-
119986-58-2
N-(4-chlorophenyl)-N-cyano-4-methylbenzenesulfonamide

-
-
1692-25-7
3-pyridylboronic acid

-
A
-
100-54-9
pyridine-3-carbonitrile

-
B
-
2903-34-6
4-methyl-N-(4-chlorophenyl)benzenesulfonamide

| Conditions | Yield |
|---|---|
| With Fe3O4/SiO2/(3-chloropropyl)trimethoxysilane/2,2′-(4,4′-(propane-1,3-diyl)bis(piperazine-4,1-diyl))- diethanamine/Pd In acetonitrile for 17h; Catalytic behavior; Reflux; | A 92% B n/a |


| Conditions | Yield |
|---|---|
| With dichloro[bis{1-(dicyclohexylphosphanyl)piperidine}]palladium; sodium carbonate In 1-methyl-pyrrolidin-2-one at 140℃; for 18h; Inert atmosphere; | 91% |
| With caesium carbonate In N,N-dimethyl-formamide at 130℃; for 8h; Inert atmosphere; Sealed tube; | 91% |
| With 5,5’-(1,2-phenylene)bis(1H-tetrazole); copper(I) iodide; caesium carbonate; potassium iodide In N,N-dimethyl-formamide at 130℃; for 10h; Inert atmosphere; | 90% |

-
-
1692-25-7
3-pyridylboronic acid

-
A
-
100-54-9
pyridine-3-carbonitrile

-
B
-
16937-03-4
4-nitro-N-(4-chlorophenyl)benzenesulfonamide

| Conditions | Yield |
|---|---|
| With Fe3O4/SiO2/(3-chloropropyl)trimethoxysilane/2,2′-(4,4′-(propane-1,3-diyl)bis(piperazine-4,1-diyl))- diethanamine/Pd In acetonitrile for 11h; Catalytic behavior; Reflux; | A 91% B n/a |


| Conditions | Yield |
|---|---|
| With ammonium carbonate; diphosphorus tetraiodide In carbon disulfide at 20℃; for 5h; | 90% |
| With hydroxyammonium sulfate; zinc for 0.416667h; Microwave irradiation; | 89% |
| With aluminum oxide; aminosulfonic acid; urea for 0.2h; Irradiation; | 77% |

| Conditions | Yield |
|---|---|
| With water; oxygen at 140℃; under 3800.26 Torr; for 24h; | A n/a B 90% |
| With ammonia; oxygen; vanadia at 375℃; Product distribution; other catalysts, effect of the presence of O2; | |
| With ammonia In 1,4-dioxane; water at 130℃; under 4560.31 Torr; for 3h; Autoclave; | A 7 %Chromat. B 84 %Chromat. |

-
-
1692-25-7
3-pyridylboronic acid

-
A
-
100-54-9
pyridine-3-carbonitrile

-
B
-
6295-97-2
4-bromo-benzenesulfonic acid-(4-chloro-anilide)

| Conditions | Yield |
|---|---|
| With Fe3O4/SiO2/(3-chloropropyl)trimethoxysilane/2,2′-(4,4′-(propane-1,3-diyl)bis(piperazine-4,1-diyl))- diethanamine/Pd In acetonitrile for 15h; Catalytic behavior; Reflux; | A 90% B n/a |


| Conditions | Yield |
|---|---|
| With sodium azide; tris(o-methoxyphenyl)phosphine; palladium dichloride In acetone at 70℃; for 36h; Schlenk technique; | 89% |
| With sodium azide; palladium diacetate; XPhos In acetone at 80℃; for 12h; Inert atmosphere; | 70% |

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0) In N,N-dimethyl-formamide for 0.0333333h; Substitution; microwave irradiation; | 88% |
| With 1,1'-bis-(diphenylphosphino)ferrocene; zinc diacetate; zinc; tris(dibenzylideneacetone)dipalladium (0) In water; N,N-dimethyl-formamide at 90 - 100℃; for 3h; | 80% |
| With water; tri tert-butylphosphoniumtetrafluoroborate; zinc; tris(dibenzylideneacetone)dipalladium(0) chloroform complex In 1-methyl-pyrrolidin-2-one at 80℃; for 3h; | 40% |
| With bromine; triphenylphosphine; zinc; palladium on activated charcoal In dimethyl amine at 80℃; for 5h; | 75 % Chromat. |
| With bromine; triphenylphosphine; zinc; palladium on activated charcoal In N,N-dimethyl acetamide at 80℃; for 5h; | 75 % Chromat. |
-
-
51892-16-1
nicotinaldehyde oxime

-
-
100-54-9
pyridine-3-carbonitrile

| Conditions | Yield |
|---|---|
| With [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; 4 A molecular sieve In acetonitrile at 80℃; for 1h; | 88% |
| With palladium diacetate; triphenylphosphine In acetonitrile for 7h; Reflux; | 87% |
| With per-rhenic acid In water; 1,3,5-trimethyl-benzene for 72h; Heating; | 84% |
| With per-rhenic acid In water; 1,3,5-trimethyl-benzene for 3h; Heating; | 84% |
| With N,N-dimethyl-formamide at 135℃; for 48h; | 83% |

| Conditions | Yield |
|---|---|
| With 1,1'-bis-(diphenylphosphino)ferrocene; palladium 10% on activated carbon; zinc(II) formate dihydrate In N,N-dimethyl acetamide at 100℃; for 12h; Inert atmosphere; | 88% |

| Conditions | Yield |
|---|---|
| With bis(trimethylsilyl)amide yttrium(III) In toluene at 20℃; for 12h; Inert atmosphere; | A 87% B n/a |

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran at -78℃; for 0.5h; cyanation; | 86% |

| Conditions | Yield |
|---|---|
| With sodium carbonate; palladium diacetate at 140℃; for 16h; | 86% |
| With sodium carbonate; potassium iodide; N,N`-dimethylethylenediamine; copper(II) bis(tetrafluoroborate) In N,N-dimethyl acetamide at 140℃; for 16h; | 67% |
| With sodium carbonate; palladium diacetate at 140℃; for 16h; | |
| With palladium diacetate; sodium carbonate In N,N-dimethyl acetamide at 120℃; for 8h; | 86 % Chromat. |

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride; sodium methylate In methanol Heating; | 100% |
| With hydroxylamine hydrochloride; sodium hydrogencarbonate In isopropyl alcohol at 80 - 85℃; | 91% |
| With hydroxylamine hydrochloride In ethanol for 8h; Reflux; | 89% |

| Conditions | Yield |
|---|---|
| With manganese(IV) oxide; silica gel In hexane for 8h; Heating; | 100% |
| With manganese(IV) oxide; silica gel In chlorobenzene for 5h; Heating; | 100% |
| With water at 30℃; for 5.5h; Large scale reaction; Enzymatic reaction; | 99% |
-
-
100-54-9
pyridine-3-carbonitrile

-
-
358-23-6
trifluoromethylsulfonic anhydride

-
-
4481-35-0
cyclohepta[b]furan-2(2H)-one

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; | 100% |

-
-
100-54-9
pyridine-3-carbonitrile

-
-
84359-15-9
(pyridin-3-yl)methylamine hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride; hydrogen In 1,4-dioxane; methanol at 60℃; under 375.038 Torr; for 18h; Flow reactor; | 100% |
| Stage #1: pyridine-3-carbonitrile With hydrogen at 130℃; under 750.075 Torr; for 6h; Stage #2: Acidic conditions; chemoselective reaction; | 99% |
| With fac-[(CO)3Mn(iPr2P(CH2)2PiPr2)(triflato)]; potassium tert-butylate; hydrogen In iso-butanol at 110℃; under 25858.1 Torr; for 0.25h; Catalytic behavior; Glovebox; Schlenk technique; Inert atmosphere; Autoclave; | 96% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In water; dimethyl sulfoxide at 60℃; for 8h; High pressure; Green chemistry; | 99.9% |
| With C13H26B(1-)*K(1+) In tetrahydrofuran for 24h; Ambient temperature; | 96% |
| Stage #1: pyridine-3-carbonitrile With diisobutylaluminium hydride In toluene at 20℃; for 0.222222h; Flow reactor; Stage #2: With water; sodium L-tartrate In toluene at 0℃; chemoselective reaction; | 52% |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In water; dimethyl sulfoxide at 60℃; for 6h; High pressure; Green chemistry; | 99.9% |
| With palladium 10% on activated carbon; ammonia; hydrogen In methanol at 30℃; under 7500.75 Torr; for 4h; Reagent/catalyst; Temperature; Pressure; Autoclave; | 93.44% |
| With hydrogen; silica gel; nickel In methanol; ammonia at 120℃; under 7600 Torr; for 6h; | 86% |
-
-
100-54-9
pyridine-3-carbonitrile

-
-
14906-64-0
3-cyanopyridine N-oxide

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; VxSi4xO6.4x In acetonitrile at 80℃; for 3h; | 99% |
| With phosphomolybdic acid; dihydrogen peroxide In water at 50 - 65℃; for 3h; | 98% |
| With titanium silicate; dihydrogen peroxide In methanol at 60℃; for 25h; Oxidation; | 97.3% |
-
-
100-54-9
pyridine-3-carbonitrile

-
-
14104-20-2
silver tetrafluoroborate

-
-
2966-50-9
silver trifluoroacetate

| Conditions | Yield |
|---|---|
| In water High Pressure; excess moistened Ag2C2 was added to concd. aq. soln. CF3COOAg anf AgBF4,soln. was filtered, 3-cyanopyridine was added, suspn. was placed in Tef lon-lined stainless steel reaction vessel and heated at 108°C for40 h; react. mixt was cooled to room temp. (6°C/h); elem. anal.; | 99% |


| Conditions | Yield |
|---|---|
| In methanol; water; acetonitrile powder X-ray diffraction; | 99% |
| In methanol; water; acetonitrile soln. 3-CNpy and Na(N(CN)2) in MeCN-MeOH was heated for 5 min, aq. soln.Fe(ClO4)2*6H2O was added; soln. was filtered and left to stand at room temp. for 3 weeks; elem. anal.; | 15% |

-
-
100-54-9
pyridine-3-carbonitrile

-
-
1428419-75-3
4-(4-(dimethylamino)phenyl)nicotinonitrile

| Conditions | Yield |
|---|---|
| Stage #1: pyridine-3-carbonitrile With boron trifluoride diethyl etherate In tetrahydrofuran at 0℃; for 0.25h; Inert atmosphere; Stage #2: 4-(N,N-dimethylamino)phenylmagnesium bromide lithium chloride complex In tetrahydrofuran at -30℃; for 2h; Inert atmosphere; Stage #3: With chloranil In tetrahydrofuran at 20℃; for 12h; Inert atmosphere; regioselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: pyridine-3-carbonitrile With boron trifluoride diethyl etherate In tetrahydrofuran at 0℃; for 0.25h; Inert atmosphere; Stage #2: 1-bromo-octane With tert-butylmagnesium chloride; magnesium; lithium chloride; zinc(II) chloride In tetrahydrofuran at -50 - 25℃; for 1h; Inert atmosphere; Stage #3: With chloranil In tetrahydrofuran at 20℃; Inert atmosphere; regioselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| In acetonitrile at 80℃; | 99% |
-
-
100-54-9
pyridine-3-carbonitrile

| Conditions | Yield |
|---|---|
| With hydrogen; Raney nickel In methanol; water | 98.6% |

| Conditions | Yield |
|---|---|
| With diammonium sulfide In methanol at 80℃; for 0.25h; microwave irradiation; | 98% |
| With hydrogenchloride; thiophosphate sodium salt hydrate In water; water-d2 at 50℃; for 24h; pH=6.5; Sealed tube; | 97% |
| With diammonium sulfide In methanol at 20℃; for 18h; | 96.4% |
-
-
100-54-9
pyridine-3-carbonitrile

-
-
100-39-0
benzyl bromide

-
-
6516-53-6
N-benzyl-3-cyanopyridin-1-ium bromide

| Conditions | Yield |
|---|---|
| In acetonitrile for 18h; Reflux; | 98% |
| In acetonitrile at 80℃; | 96% |
| In nitrobenzene at 40℃; Rate constant; |
-
-
100-54-9
pyridine-3-carbonitrile

-
-
4815-24-1
2-amino-4,5-dimethyl-3-thiophenecarboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With hydrogenchloride In 1,4-dioxane at 0℃; for 10h; Heating; | 98% |
| With hydrogenchloride In 1,4-dioxane for 5h; | 66% |

| Conditions | Yield |
|---|---|
| With sodium azide In N,N-dimethyl-formamide for 24h; Reflux; | 98% |
| With sodium azide In N,N-dimethyl-formamide at 110 - 120℃; for 24h; | 98% |
| With sodium azide; 1-ethyl-3-methylimidazolium hydrogensulfate at 100℃; for 9h; | 96% |

| Conditions | Yield |
|---|---|
| With Iron(III) nitrate nonahydrate at 125℃; for 24h; Neat (no solvent); Sealed tube; | 98% |
-
-
100-54-9
pyridine-3-carbonitrile

-
-
156-87-6
propan-1-ol-3-amine

-
-
1235527-97-5
2-(pyridin-3-yl)-5,6-dihydro-4H-1,3-oxazine

| Conditions | Yield |
|---|---|
| With montmorillonite K-10 at 30℃; for 0.166667h; Sonication; | 98% |
| With Montmorillonite K-10 at 125℃; for 1.3h; | 96% |
| With sulfur; cobalt(II) nitrate In neat (no solvent) at 90℃; for 0.05h; Reagent/catalyst; Solvent; Temperature; Time; Microwave irradiation; | 95% |
| With phosphotungstic acid at 125℃; for 2h; chemoselective reaction; | 90% |
| With sulfur In neat (no solvent) at 100℃; for 6h; Reagent/catalyst; | 88% |
-
-
100-54-9
pyridine-3-carbonitrile

-
-
4815-37-6
ethyl 2-amino-5-methyl-4-phenylthiophene-3-carboxylate

-
-
724746-10-5
6-methyl-5-phenyl-2-(pyridin-3-yl)thieno[2,3-d]pyrimidin-4(3H)-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride In 1,4-dioxane at 0℃; for 10h; Heating; | 98% |
-
-
100-54-9
pyridine-3-carbonitrile

-
-
1428419-76-4
4-mesitylnicotinonitrile

| Conditions | Yield |
|---|---|
| Stage #1: pyridine-3-carbonitrile With boron trifluoride diethyl etherate In tetrahydrofuran at 0℃; for 0.25h; Inert atmosphere; Stage #2: mesitylmagnesium bromide lithium chloride In tetrahydrofuran at -30℃; for 2h; Inert atmosphere; Stage #3: With chloranil In tetrahydrofuran at 20℃; for 12h; Inert atmosphere; regioselective reaction; | 98% |
-
-
100-54-9
pyridine-3-carbonitrile

-
-
64-17-5
ethanol

-
-
56624-12-5, 81659-46-3
ethyl (3-pyridyl)imidate dihydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In dichloromethane at 0 - 20℃; for 1h; | 97% |
| With acetyl chloride In chloroform for 25h; 0 deg C up to RT; | 92% |
| With hydrogenchloride Kuehlung; | |
| With hydrogenchloride In dichloromethane for 5h; |

| Conditions | Yield |
|---|---|
| With trichloroisocyanuric acid In neat (no solvent) at 110℃; for 0.05h; chemoselective reaction; | 97% |
| With tribromomelamine at 100℃; for 0.0833333h; Neat (no solvent); | 94% |
| With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione at 110℃; for 0.1h; chemoselective reaction; | 85% |
-
-
100-54-9
pyridine-3-carbonitrile

-
-
4506-71-2
ethyl 2-amino-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carboxylate

| Conditions | Yield |
|---|---|
| With hydrogenchloride In 1,4-dioxane at 0℃; for 10h; Heating; | 97% |
| With hydrogenchloride In 1,4-dioxane at 20℃; for 18h; | 74% |
| With hydrogenchloride In 1,4-dioxane for 5h; | 60% |
3-Cyanopyridine Chemical Properties
Molecular Structure:
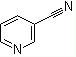
Molecular Formula: C6H4N2.
Molecular Weight: 104.11.
Density: 1.159.
Melting Point: 49 - 50 ℃.
Boiling Point: 202 ℃.
Flash Point: 85 ℃.
Solubility: 140 g/L (20℃).
3-Cyanopyridine Uses
3-Cyanopyridine Toxicity Data With Reference
| 1. | orl-rat LD50:1185 mg/kg | MEPAAX Medycyna Pracy. Industrial Medicine. 30 (1979),109. | ||
| 2. | ipr-mus LD25:810 mg/kg | JPMSAE Journal of Pharmaceutical Sciences. 64 (1975),528. | ||
| 3. | skn-rbt LDLo:4000 mg/kg | MEPAAX Medycyna Pracy. Industrial Medicine. 30 (1979),109. |
3-Cyanopyridine Consensus Reports
3-Cyanopyridine Safety Profile
Hazard Codes:
 Xn
Xn Risk Statements: 22-36/37/38
22: Harmful if swallowed
36/37/38: Irritating to eyes, respiratory system and skin
Safety Statements: 22-24/25-36/37/39-26
22: Do not breathe dust
24/25: Avoid contact with skin and eyes
36/37/39: Wear suitable protective clothing, gloves and eye/face protection
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
WGK Germany: 3
RTECS: QT3030000
HS Code: 29333999
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View