-
Name
3-Hydroxypyridine
- EINECS 203-637-4
- CAS No. 109-00-2
- Article Data121
- CAS DataBase
- Density 1.172 g/cm3
- Solubility 33g/L in water
- Melting Point 123-130 °C
- Formula C5H5NO
- Boiling Point 318.9 °C at 760 mmHg
- Molecular Weight 95.1008
- Flash Point 146.6 °C
- Transport Information
- Appearance yellow to brownish crystal
- Safety 26-36-37/39-22
- Risk Codes 36/37/38-40
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi, Xn
Xn
- Synonyms 3-Pyridol;3-Oxopyridine;.beta.-Hydroxypyridine;3-Pyridinol;3-Hydroxy Pyridine;3-Pyridone;pyridin-3-ol;
- PSA 33.12000
- LogP 0.78720
Synthetic route

| Conditions | Yield |
|---|---|
| Stage #1: pyridin-3-ylamine With sulfuric acid at 20℃; Cooling with ice; Stage #2: With sodium nitrite In water Reflux; | 100% |
| With sulfuric acid Diazotization; | |
| With sulfuric acid; water; sodium nitrite at 0℃; Erwaermen des Reaktionsgemisches auf 50grad; | |
| With sulfuric acid; water; sodium nitrite at 0℃; Erwaermen des Reaktionsgemisches auf 80grad.; |

| Conditions | Yield |
|---|---|
| With methyloxorhenium(V)(2-(mercaptomethyl)thiophenolate) triphenylphosphine; triphenylphosphine In benzene at 20℃; for 7h; | 99% |
| With triphenylphosphine; N-fused tetraphenylporphyrin rhenium(VII) trioxide In toluene at 80℃; for 4.5h; | 98% |
| With 1,1,2,2-tetrabutyl-1,2-dichloro distannane In tetrahydrofuran for 1h; Heating; | 88% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid; lithium chloride In 1-methyl-pyrrolidin-2-one at 180℃; for 1h; | 99% |
| With L-Selectride In tetrahydrofuran for 24h; Reflux; Inert atmosphere; chemoselective reaction; | 89% |
| With hexamethyldisilathiane; sodium methylate In various solvent(s) at 180℃; for 24h; | 61% |
| With trimethylammonium heptachlorodialuminate In dichloromethane Heating; |

| Conditions | Yield |
|---|---|
| With 2-di-tertbutylphosphino-3,4,5,6-tetramethyl-2',4',6'-triisopropyl-1,1'-biphenyl; potassium hydroxide; tris-(dibenzylideneacetone)dipalladium(0) In 1,4-dioxane; water at 100℃; for 10h; | 97% |
| With [(2-di-tert-butylphosphino-3-methoxy-6-methyl-2,4,6-triisopropyl-1,1-biphenyl)-2-(2-aminobiphenyl)]palladium(II) methanesulfonate; caesium carbonate; Benzaldoxime In N,N-dimethyl-formamide at 80℃; for 18h; Inert atmosphere; Glovebox; Sealed tube; | 92% |
| Stage #1: 3-Chloropyridine With sodium hydroxide In propylene glycol at 140℃; for 2h; Stage #2: With hydrogenchloride In water at 60 - 70℃; for 0.666667h; pH=6 - 7; | 90% |
| Stage #1: 3-Chloropyridine With sodium hydroxide In propylene glycol at 140℃; for 2h; Stage #2: With hydrogenchloride In methanol; water at 60 - 70℃; for 0.666667h; Temperature; | 90% |
| With copper acetylacetonate; N1-(4-hydroxy-2,6-dimethylphenyl)-N2-(4-hydroxy-3,5-dimethylphenyl)oxalamide; water In water; dimethyl sulfoxide at 130℃; for 24h; Schlenk technique; Inert atmosphere; | 80% |
-
-
17747-43-2
3-pyridyl acetate

-
-
109-00-2
3-HYDROXYPYRIDINE

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; cobalt(II) chloride In ethanol at 0 - 25℃; for 10h; | 97% |
| With Rsp3690 from Rhodobacter sphaeroides In dimethyl sulfoxide at 30℃; pH=8.3; Kinetics; Enzymatic reaction; |
-
-
50717-82-3, 74279-87-1, 74279-88-2
piperidin-3-one

-
-
109-00-2
3-HYDROXYPYRIDINE

| Conditions | Yield |
|---|---|
| With hydrogen bromide; bromine In dichloromethane at -35 - 35℃; for 5h; | 93.7% |

| Conditions | Yield |
|---|---|
| With copper(I) oxide; water; potassium carbonate; Sucrose at 140℃; for 3h; Sealed tube; Inert atmosphere; | 93% |
| With lithium salt of proline; tetrabutylammomium bromide; potassium hydroxide; copper dichloride In water at 120℃; for 0.666667h; Microwave irradiation; Green chemistry; | 83% |
| With copper acetylacetonate; N1-(4-hydroxy-2,6-dimethylphenyl)-N2-(4-hydroxy-3,5-dimethylphenyl)oxalamide; potassium hydroxide In water; dimethyl sulfoxide at 60℃; for 24h; Schlenk technique; Inert atmosphere; | 82% |
| Multi-step reaction with 2 steps 1.1: copper(l) iodide; 1,10-Phenanthroline; caesium carbonate / toluene / 14 h / 110 °C / Inert atmosphere; Sealed tube 2.1: cesium fluoride / N,N-dimethyl-formamide / 1 h / 60 °C / Inert atmosphere 2.2: Inert atmosphere View Scheme |
-
-
1338215-39-6
3-(2-trimethylsilanylethoxy)pyridine

-
-
109-00-2
3-HYDROXYPYRIDINE

| Conditions | Yield |
|---|---|
| Stage #1: 3-(2-trimethylsilanylethoxy)pyridine With cesium fluoride In N,N-dimethyl-formamide at 60℃; for 1h; Inert atmosphere; Stage #2: With water In N,N-dimethyl-formamide Inert atmosphere; | 93% |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In acetonitrile at 30 - 35℃; for 0.166667h; Schlenk technique; | 92% |
| With water; caesium carbonate; hydrazine hydrate at 80℃; for 24h; | 91% |
| With urea hydrogen peroxide adduct In acetonitrile at 27 - 29℃; for 6h; Green chemistry; chemoselective reaction; | 91% |
-
-
115437-94-0
3-(tert-butyldimethylsilyloxy)pyridine

-
-
109-00-2
3-HYDROXYPYRIDINE

| Conditions | Yield |
|---|---|
| With lithium acetate In water; N,N-dimethyl-formamide at 25℃; for 2.5h; Inert atmosphere; | 90% |
-
-
849774-31-8
3-[(triisopropylsilyl)oxy]pyridine

-
-
109-00-2
3-HYDROXYPYRIDINE

| Conditions | Yield |
|---|---|
| With potassium acetate In water; N,N-dimethyl-formamide at 25℃; for 4h; | 89% |
-
-
329214-79-1
3-(4,4,5,5,-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine

-
-
109-00-2
3-HYDROXYPYRIDINE

| Conditions | Yield |
|---|---|
| With menadione; sodium hydrogencarbonate; sodium L-ascorbate In ethanol; water at 20℃; under 760.051 Torr; for 24h; pH=8.5; Darkness; Green chemistry; | 83% |
| With 3,4,5-trihydroxybenzoic acid; sodium hydrogencarbonate In ethanol; water at 20℃; for 24h; Green chemistry; | 79% |
-
-
252974-70-2
3-(dimethylsilyl)pyridine

-
-
109-00-2
3-HYDROXYPYRIDINE

| Conditions | Yield |
|---|---|
| With water; dihydrogen peroxide; potassium hydrogencarbonate In tetrahydrofuran; methanol at 20℃; for 2h; | 78% |

| Conditions | Yield |
|---|---|
| With lithium salt of proline; tetrabutylammomium bromide; potassium hydroxide; copper dichloride In water at 120℃; for 0.666667h; Microwave irradiation; Green chemistry; | 75% |
| With copper(l) iodide; lithium pipecolinate; tetrabutyl ammonium fluoride; sodium hydroxide In water at 130℃; for 24h; | 74% |
| With potassium phosphate; copper(l) iodide; water; N,N`-dimethylethylenediamine at 20 - 180℃; for 0.5h; Microwave irradiation; | 70% |
-
-
874-24-8
3-hydroxypyridine-2-carboxylic acid

-
-
611-10-9
2-ethoxycarbonyl-1-cyclopentanone

-
A
-
109-00-2
3-HYDROXYPYRIDINE

| Conditions | Yield |
|---|---|
| at 160℃; for 6h; | A 74% B 25% |

-
-
874-24-8
3-hydroxypyridine-2-carboxylic acid

-
-
5394-63-8
2,2,6-trimethyl-4H-1,3-dioxin-4-one

-
A
-
109-00-2
3-HYDROXYPYRIDINE

-
B
-
771-03-9
3-acetyl-4-hydroxy-6-methyl-2H-pyran-2-one

| Conditions | Yield |
|---|---|
| In toluene for 6h; Mechanism; Heating; other 3-substituted picolinic acids; other acetylketenes; var. temperatures and solvents; | A 70% B 29.7% C 25% |
| In toluene for 6h; Heating; | A 70% B 29.7% C 25% |

-
-
5394-63-8
2,2,6-trimethyl-4H-1,3-dioxin-4-one

-
A
-
109-00-2
3-HYDROXYPYRIDINE

-
B
-
771-03-9
3-acetyl-4-hydroxy-6-methyl-2H-pyran-2-one

| Conditions | Yield |
|---|---|
| With 3-hydroxypyridine-2-carboxylic acid In toluene for 6h; Heating; | A 70% B 29.7% C 25% |


| Conditions | Yield |
|---|---|
| With sulfuric acid; mercury(II) sulfate at 210℃; for 10h; Temperature; | 62% |
| Multi-step reaction with 2 steps 1: concentrated sulfuric acid 2: bei der Kalischmelze View Scheme |

-
-
109-00-2
3-HYDROXYPYRIDINE

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene; water; triethylamine In acetonitrile at 20℃; for 1h; | 59% |
| With sodium periodate; iodobenzene In water; acetonitrile at 80℃; for 8h; | 59% |
-
-
39871-47-1
α-azidocyclopentanone

-
A
-
109-00-2
3-HYDROXYPYRIDINE

-
B
-
57147-25-8
2-oxo-1,2,3,4-tetrahydropyridine

| Conditions | Yield |
|---|---|
| at 600℃; under 0.01 Torr; for 1h; Flash photolysis; | A 7% B 55% |
-
-
74115-13-2
5-bromopyridine-3-ol

-
-
109-72-8, 29786-93-4
n-butyllithium

-
-
1066-45-1
trimethyltin(IV)chloride

-
A
-
109-00-2
3-HYDROXYPYRIDINE

-
B
-
142929-09-7
<3,3'-Bipyridin>-5,5'-diol

-
D
-
918631-14-8
5-trimethylstannanyl-pyridin-3-ol

| Conditions | Yield |
|---|---|
| Stage #1: 5-bromopyridine-3-ol; n-butyllithium In tetrahydrofuran at -78℃; Stage #2: trimethyltin(IV)chloride In tetrahydrofuran | A 24% B 29% C 11% D 33% |

-
-
2168-13-0
2-[(dimethylamino)methyl]pyridin-3-ol

-
-
62-53-3
aniline

-
A
-
109-00-2
3-HYDROXYPYRIDINE

-
B
-
65974-47-2
2-[(phenylimino)methyl]-1-azabenzene-3-ol

-
C
-
74803-54-6
2-[(phenylamino)methyl]-1-azabenzene-3-ol

| Conditions | Yield |
|---|---|
| In diphenylether at 250℃; for 15h; | A 30% B 10% C 20% |


| Conditions | Yield |
|---|---|
| In benzene for 3h; Irradiation; Inert atmosphere; | A 15% B 9% |
-
-
110-89-4
piperidine

-
-
99073-54-8
(5-nitro-[2]pyridyl)-[3]pyridyl ether

-
A
-
109-00-2
3-HYDROXYPYRIDINE

-
B
-
26820-61-1
5-nitro-2-piperidinopyridine


| Conditions | Yield |
|---|---|
| With ammonium sulfate; water; urea at 160℃; unter Druck; | |
| With ammonium sulfate; ammonium oxalate; water at 160℃; unter Druck; | |
| With ammonium chloride In methanol; aq. phosphate buffer at 100℃; for 22h; pH=8; Time; Temperature; Sealed tube; |

| Conditions | Yield |
|---|---|
| With ethanol; water; hydrazinium sulfate at 153℃; unter Druck; |
-
-
14773-50-3
3-ethoxypyridine

-
-
109-00-2
3-HYDROXYPYRIDINE

| Conditions | Yield |
|---|---|
| With hydrogen iodide at 110 - 120℃; |
-
-
15069-92-8
5-hydroxypicolinic acid

-
-
109-00-2
3-HYDROXYPYRIDINE

| Conditions | Yield |
|---|---|
| With calcium oxide | |
| at 300℃; unter vermindertem Druck; |

| Conditions | Yield |
|---|---|
| bei der trocknen Destillation; |
-
-
109-00-2
3-HYDROXYPYRIDINE

-
-
1121-76-2
4-chloropyridine N-oxide

-
-
75734-24-6
3-hydroxy-1-(1-oxido-4-pyridyl)pyridinium chloride

| Conditions | Yield |
|---|---|
| In chlorobenzene for 12h; Heating; | 100% |
-
-
109-00-2
3-HYDROXYPYRIDINE

-
-
103057-44-9
N-(tert-butoxycarbonyl)-3-hydroxypyrrolidine

-
-
224818-73-9
(+/-)-3-oxy(3-pyridyl)-1-tert-butoxycarbonylpyrrolidine

| Conditions | Yield |
|---|---|
| With triphenylphosphine; diethylazodicarboxylate In tetrahydrofuran at 40℃; for 15h; Condensation; | 100% |
| With triphenylphosphine; diethylazodicarboxylate In tetrahydrofuran Mitsunobu reaction; | |
| With triphenylphosphine; diethylazodicarboxylate In tetrahydrofuran | |
| Stage #1: With triphenylphosphine; diethylazodicarboxylate In tetrahydrofuran at -20℃; for 0.166667h; Stage #2: N-(tert-butoxycarbonyl)-3-hydroxypyrrolidine In tetrahydrofuran at -20℃; for 0.166667h; Stage #3: 3-HYDROXYPYRIDINE In tetrahydrofuran at 20℃; Mitsunobu reaction; | |
| With triphenylphosphine; diethylazodicarboxylate In tetrahydrofuran at 0 - 20℃; Mitsunobu reaction; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 30℃; Product distribution; Kinetics; Further Variations:; Solvents; | 100% |
| Stage #1: 3-HYDROXYPYRIDINE With sodium hydride In tetrahydrofuran for 0.166667h; Stage #2: 2,4,6-trinitrochlorobenzene In tetrahydrofuran for 0.166667h; |
-
-
109-00-2
3-HYDROXYPYRIDINE

-
-
100-39-0
benzyl bromide

-
-
61995-15-1
3-benzyloxy-N-benzyl-1,2,5,6-tetrahydropyridine

| Conditions | Yield |
|---|---|
| Stage #1: 3-HYDROXYPYRIDINE; benzyl bromide With sodium methylate In methanol Heating / reflux; Stage #2: With sodium tetrahydroborate In methanol at 20℃; Stage #3: With potassium carbonate In diethyl ether; water for 1h; | 100% |
-
-
109-00-2
3-HYDROXYPYRIDINE

-
-
59142-68-6
2-bromo-4-fluorobenzaldehyde

-
-
1196474-75-5
2-bromo-4-(pyridine-3-yloxy)-benzaldehyde

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 16h; | 100% |
-
-
109-00-2
3-HYDROXYPYRIDINE

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 70℃; for 17h; Inert atmosphere; | 100% |
| With caesium carbonate In N,N-dimethyl-formamide at 70℃; for 17h; Inert atmosphere; | 100% |
-
-
109-00-2
3-HYDROXYPYRIDINE

-
-
1246303-46-7
dihydroxo[5,10,15,20-tetrakis(2-thienyl)porphyrinato]tin(IV)

| Conditions | Yield |
|---|---|
| In chloroform for 4h; Reflux; | 100% |
-
-
109-00-2
3-HYDROXYPYRIDINE

-
-
26334-89-4
trans-dihydroxo(meso-tetraphenylporphyrinato)tin(IV)

-
-
1365636-06-1
trans-bis(phenolato)-[5,10,15,20-tetrakis(phenyl)porphyrinato]tin(IV)

| Conditions | Yield |
|---|---|
| In chloroform for 4h; Reflux; | 100% |
-
-
109-00-2
3-HYDROXYPYRIDINE

-
-
100-39-0
benzyl bromide

-
-
62214-78-2
1-benzyl-3-hydroxypyridin-1-ium bromide

| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 16h; Reflux; Inert atmosphere; | 99% |
| In acetone Ambient temperature; | 97% |
| With toluene |

| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 16h; Reflux; Inert atmosphere; | 99% |
| In toluene Reflux; | 89% |
| In methanol for 6h; Heating; | 86% |
-
-
109-00-2
3-HYDROXYPYRIDINE

-
-
3433-80-5
1-Bromo-2-bromomethyl-benzene

-
-
123100-48-1
1-(2-bromobenzyl)-3-hydroxypyridin-1-ium bromide

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 16h; Reflux; Inert atmosphere; | 99% |
| In tetrahydrofuran for 2.5h; Heating; | 80% |

| Conditions | Yield |
|---|---|
| With magnesium(II) perchlorate at 40℃; for 5.5h; | 99% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water | 99% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 30℃; for 0.5h; Reagent/catalyst; Solvent; Temperature; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 16h; Reflux; Inert atmosphere; | 99% |
-
-
109-00-2
3-HYDROXYPYRIDINE

-
-
61150-57-0
2-bromo-4-fluorobenzyl bromide

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 16h; Reflux; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With aluminum oxide; 1.5% Rh/SiO2; hydrogen In water at 85℃; under 52505.3 Torr; Reagent/catalyst; Pressure; Temperature; Autoclave; | 98.7% |
| With hydrogen; Rh on carbon In water at 80℃; under 3800 Torr; for 2h; | 96% |
| With 10% Rh/C; hydrogen In water at 80℃; under 3800.26 Torr; for 2h; | 96% |
-
-
109-00-2
3-HYDROXYPYRIDINE

-
-
74780-46-4
trans-1-(2-chloro-5-nitrophenyl)-3-chloroprop-2-en-1-one

-
-
74780-45-3
1--3-hydroxypyridinium chloride

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 3h; | 98% |
-
-
109-00-2
3-HYDROXYPYRIDINE

-
-
26127-08-2
(1R,2S,5R)-1-(chloromethoxy)-2-isopropyl-5-methylcyclohexane

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 20℃; Menschutkin quaternization; | 98% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In dichloromethane; water at 20℃; for 3h; | 98% |
-
-
109-00-2
3-HYDROXYPYRIDINE

-
-
1202403-70-0
3-(2-chloroacetamido)-7,8-dehydrorutaecarpine

-
-
1443150-25-1
3-(2-(pyridin-3-yloxy)-acetamino)-7,8-dehydrorutaecarpine

| Conditions | Yield |
|---|---|
| for 8h; Reflux; | 98% |
-
-
109-00-2
3-HYDROXYPYRIDINE

| Conditions | Yield |
|---|---|
| Stage #1: 3-HYDROXYPYRIDINE With triethylamine In dichloromethane at 20℃; for 0.166667h; Stage #2: With fluorosulfonyl fluoride In dichloromethane at 20℃; for 2h; chemoselective reaction; | 98% |
| With fluorosulfonyl fluoride; triethylamine In dichloromethane at 20℃; | 95% |
| With fluorosulfonyl fluoride; triethylamine In dichloromethane at 20℃; under 760.051 Torr; for 24h; | 80% |

| Conditions | Yield |
|---|---|
| In acetonitrile for 48h; Menshutkin Reaction; Reflux; | 98% |

| Conditions | Yield |
|---|---|
| In acetonitrile for 48h; Menshutkin Reaction; Reflux; | 98% |
-
-
109-00-2
3-HYDROXYPYRIDINE

-
-
1258190-63-4
S-methyl pyridine-3-dithiocarbamate

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 0℃; for 1h; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| at 110℃; | 97% |
| In toluene for 1h; Alkylation; Heating; | 94% |
| In toluene for 1h; Heating; | 94% |
-
-
109-00-2
3-HYDROXYPYRIDINE

-
-
107658-27-5
pyridin-3-yl trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 8h; Acylation; | 97% |
3-Hydroxypyridine Consensus Reports
3-Hydroxypyridine Specification
The 3-Pyridinol, with the CAS registry number 109-00-2, is also known as beta-Hydroxypyridine. It belongs to the product categories of Pyridine; Pyridines, Pyrimidines, Purines and Pteredines; Pyridines Derivatives; Pyridines Derivates. Its EINECS registry number is 203-637-4. This chemical's molecular formula is C5H5NO and molecular weight is 95.0993. Its IUPAC name is called pyridin-3-ol. What's more, this chemical's classification codes are Antioxidants; Protective Agents. It is white to light yellow crystal which is used in organic synthesis, medicine and dye preparation.
Physical properties of 3-Pyridinol: (1)ACD/LogP: 0.64; (2)ACD/LogD (pH 5.5): 0.35; (3)ACD/LogD (pH 7.4): 0.41; (4)ACD/BCF (pH 5.5): 1; (5)ACD/BCF (pH 7.4): 1.06; (6)ACD/KOC (pH 5.5): 27.32; (7)ACD/KOC (pH 7.4): 31.28; (8)#H bond acceptors: 2; (9)#H bond donors: 1; (10)#Freely Rotating Bonds: 1; (11)Index of Refraction: 1.56; (12)Molar Refractivity: 26.22 cm3; (13)Molar Volume: 81 cm3; (14)Surface Tension: 50.7 dyne/cm; (15)Density: 1.172 g/cm3; (16)Flash Point: 146.6 °C; (17)Enthalpy of Vaporization: 58.27 kJ/mol; (18)Boiling Point: 318.9 °C at 760 mmHg; (19)Vapour Pressure: 0.000188 mmHg at 25°C.
Preparation of 3-Pyridinol: this chemical can be prepared by 3-methoxy-pyridine. This reaction will need reagents hexamethyldisilathiane, sodium methoxide and various solvents. The reaction time is 24 hours with reaction temperature of 180 °C. The yield is about 61%.

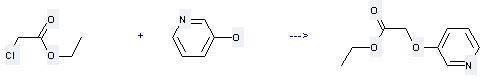
Uses of 3-Pyridinol: it can be used to produce pyridin-3-yloxy-acetic acid ethyl ester with chloroacetic acid ethyl ester by heating. This reaction will need reagent K2CO3 and solvent acetone with reaction time of 20 hours. The yield is about 88%.
When you are using this chemical, please be cautious about it as the following:
This chemical may cause inflammation to the skin or other mucous membranes. It is irritating to eyes, respiratory system and skin. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. Whenever you will contact it, please wear suitable protective clothing, gloves and eye/face protection.
You can still convert the following datas into molecular structure:
(1)Canonical SMILES: C1=CC(=CN=C1)O
(2)InChI: InChI=1S/C5H5NO/c7-5-2-1-3-6-4-5/h1-4,7H
(3)InChIKey: GRFNBEZIAWKNCO-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| bird - wild | LD50 | oral | 750mg/kg (750mg/kg) | Archives of Environmental Contamination and Toxicology. Vol. 12, Pg. 355, 1983. | |
| mammal (species unspecified) | LD50 | unreported | 900mg/kg (900mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) | Pharmaceutical Chemistry Journal Vol. 16, Pg. 259, 1982. |
| mouse | LD50 | intraperitoneal | 1822mg/kg (1822mg/kg) | Toxicon. Vol. 23, Pg. 815, 1985. |
Related Products
- 3-Hydroxypyridine
- 3-Hydroxypyridine sodium salt
- 3-Hydroxypyridine-2-carboxaldehyde
- 3-Hydroxypyridine-2-carboxylic acid
- 3-Hydroxypyridine-2-carboxylic acid methyl ester
- 3-Hydroxypyridine-4-carboxaldehyde
- 3-Hydroxypyridine-4-carboxylic acid
- 3-Hydroxypyridine-N-oxide
- 109008-26-6
- 109010-10-8
- 109010-60-8
- 109012-23-9
- 109-01-3
- 1090-13-7
- 109021-59-2
- 109-02-4
- 109025-88-9
- 109029-21-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View