-
Name
INDOXYL ACETATE
- EINECS 210-154-2
- CAS No. 608-08-2
- Article Data16
- CAS DataBase
- Density 1.255 g/cm3
- Solubility Soluble in ethanol, water, chloroform, dichloromethane and methanol.
- Melting Point 128-130 °C(lit.)
- Formula C10H9NO2
- Boiling Point 339.1 °C at 760 mmHg
- Molecular Weight 175.187
- Flash Point 158.9 °C
- Transport Information
- Appearance Dark blue solid
- Safety 26-36
- Risk Codes 22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms 1H-Indol-3-ol,acetate (ester) (9CI);Indol-3-ol, acetate (7CI);Indol-3-ol, acetate (ester)(8CI);Indoxyl acetate (6CI);3-Acetoxyindole;3-Indolyl acetate;3-Indoxylacetate;NSC 13964;
- PSA 42.09000
- LogP 2.09320
Synthetic route

| Conditions | Yield |
|---|---|
| With potassium hydroxide In acetonitrile at 35℃; for 1.5h; | 76% |
| With potassium hydroxide In toluene; acetonitrile at 20℃; for 1.5h; Green chemistry; |

| Conditions | Yield |
|---|---|
| In dichloromethane for 0.133333h; Irradiation; | A 74% B n/a |

| Conditions | Yield |
|---|---|
| With acetic acid at 30℃; for 2h; | 43% |

| Conditions | Yield |
|---|---|
| With acetic acid at 90℃; for 1h; | 41% |
| With acetic acid |
-
-
100063-39-6
2-Acetyl-1H-indol-3-yl-acetat

-
-
608-08-2
3-indoxyl acetate

| Conditions | Yield |
|---|---|
| With sodium hydroxide; water unter Stickstoff und Behandeln des Reaktionsprodukts mit Acetanhydrid bei 0grad; |

| Conditions | Yield |
|---|---|
| With potassium hydroxide at 260℃; Behandeln des mit CO2 und Essigsaeure teilweise neutralisierten Reaktionsgemisches mit Acetanhydrid; |

| Conditions | Yield |
|---|---|
| With sulfuric acid; water | |
| With alkali | |
| With alkali |
-
-
140843-81-8
1-Nitrosoindol-3-yl acetate

-
-
608-08-2
3-indoxyl acetate

| Conditions | Yield |
|---|---|
| In 1,4-dioxane; water at 25℃; Equilibrium constant; |
-
-
67790-18-5
3-methoxycarbonyl-5H-1,2-benzodiazepine

-
-
608-08-2
3-indoxyl acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 68 percent / CH2Cl2 / 0 - 20 °C 2: 74 percent / CH2Cl2 / 0.13 h / Irradiation View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: water / 70 - 80 °C 2: alkali View Scheme | |
| Multi-step reaction with 2 steps 2: alkali View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: methanol. NaOH-solution; iodine; potassium iodide 2: acetic acid View Scheme |

| Conditions | Yield |
|---|---|
| Stage #1: 3-indoxyl acetate; N-Methyl-7-azaisatin In methanol at 25℃; Inert atmosphere; Stage #2: With sodium carbonate In methanol at 25℃; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With sodium carbonate In methanol at 20℃; for 3h; | 95% |
| With sodium carbonate In methanol at 20℃; | |
| With sodium carbonate |

| Conditions | Yield |
|---|---|
| With sodium carbonate In methanol at 20℃; for 3h; | 95% |
| With sodium carbonate In methanol for 3.5h; | 80% |
-
-
608-08-2
3-indoxyl acetate

-
-
63220-48-4
7-chloro-1-methylindoline-2,3-dione

-
-
906660-40-0
7-Chloro-1-methylindirubin

| Conditions | Yield |
|---|---|
| With sodium carbonate In methanol | 95% |

| Conditions | Yield |
|---|---|
| With sodium carbonate In methanol at 20℃; for 3h; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| With sodium carbonate In methanol for 48h; | 94% |
| With potassium carbonate In methanol at 20℃; for 25h; | 85% |
| With sodium carbonate In methanol at 20℃; for 24.5h; | 81% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In methanol at 20℃; for 5h; Inert atmosphere; | 93% |
| With sodium carbonate In methanol at 25℃; for 36h; | 82% |
| With sodium carbonate In methanol at 20℃; | 73% |

| Conditions | Yield |
|---|---|
| With sodium carbonate In methanol at 20℃; for 3h; Inert atmosphere; | 93% |
| Multi-step reaction with 2 steps 1: sodium carbonate / methanol / 20 °C 2: hydroxylamine hydrochloride; pyridine / 2 h / 120 °C View Scheme | |
| Multi-step reaction with 2 steps 1: sodium carbonate / methanol 2: hydroxylamine hydrochloride; pyridine / Heating View Scheme |

| Conditions | Yield |
|---|---|
| With sodium carbonate In methanol at 20℃; | 93% |
-
-
608-08-2
3-indoxyl acetate

-
-
25128-32-9
1H-indole-2,3-dione-5-carboxylic acid

-
-
1238056-78-4
indirubin-5-carboxylic acid

| Conditions | Yield |
|---|---|
| With sodium carbonate In methanol at 20℃; for 20h; Inert atmosphere; | 92% |
-
-
608-08-2
3-indoxyl acetate

-
-
343-69-1
6-(Trifluoromethyl)-1H-indol-2,3-dione

-
-
1257060-35-7
6-trifluoromethylindirubin

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In ethanol at 65 - 70℃; for 3h; | 91% |

| Conditions | Yield |
|---|---|
| With sodium carbonate In methanol at 20℃; | 91% |
| With sodium carbonate In methanol | |
| With sodium carbonate In methanol |

| Conditions | Yield |
|---|---|
| With sodium carbonate In methanol at 20℃; for 3h; | 90% |
| Stage #1: 5-Aminoisatin; 3-indoxyl acetate In methanol at 25℃; for 0.416667h; Stage #2: With sodium carbonate In methanol at 25℃; for 3h; | 90% |
| With sodium carbonate In methanol at 45℃; for 1h; Inert atmosphere; | 90% |
-
-
608-08-2
3-indoxyl acetate

-
-
75591-28-5
N-(2,3-dioxoindolin-5-yl)acetamide

-
-
910035-28-8
5-Acetylaminoindirubin

| Conditions | Yield |
|---|---|
| With sodium carbonate In methanol at 20℃; for 3h; | 90% |
| With sodium carbonate In methanol at 45℃; for 1h; Inert atmosphere; | 90% |

| Conditions | Yield |
|---|---|
| With sodium carbonate In methanol at 20℃; for 3h; | 90% |

| Conditions | Yield |
|---|---|
| With sodium carbonate In methanol | 90% |

| Conditions | Yield |
|---|---|
| Stage #1: 6-bromoisatin; 3-indoxyl acetate In methanol for 0.416667h; Stage #2: With sodium carbonate In methanol for 3h; | 90% |
| With sodium carbonate In methanol at 25℃; for 5h; Inert atmosphere; Darkness; | 90% |
| With toluene-4-sulfonic acid In ethanol at 65 - 70℃; for 3h; | 87% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-indoxyl acetate; 6-bromo-5-aminoisatin In methanol at 25℃; for 0.416667h; Stage #2: With sodium carbonate In methanol at 25℃; for 3h; | 90% |

| Conditions | Yield |
|---|---|
| With sodium carbonate In methanol at 20℃; for 3h; | 89% |
| With sodium carbonate In methanol at 45℃; for 1h; Inert atmosphere; | 89% |
| With sodium carbonate In methanol at 20℃; | |
| With sodium carbonate In methanol at 20℃; | |
| With sodium carbonate |

| Conditions | Yield |
|---|---|
| With sodium carbonate In methanol at 20℃; for 3h; Inert atmosphere; | 89% |
| With sodium carbonate In methanol at 20℃; Inert atmosphere; | 85% |
| With sodium carbonate In methanol | |
| In methanol Alkaline conditions; | |
| With sodium carbonate In methanol |

| Conditions | Yield |
|---|---|
| With sodium carbonate In methanol at 20℃; | 88% |
-
-
906660-36-4
7-iodo-1-methylindolin-2,3-dione

-
-
608-08-2
3-indoxyl acetate

-
-
906660-42-2
7-Iodo-1-methylindirubin

| Conditions | Yield |
|---|---|
| With sodium carbonate In methanol | 87% |
-
-
608-08-2
3-indoxyl acetate

-
-
100-10-7
4-dimethylamino-benzaldehyde

-
-
38072-53-6
(Z)-2-(4-(dimethylamino)benzylidene)indolin-3-one

| Conditions | Yield |
|---|---|
| Stage #1: 3-indoxyl acetate With sodium hydroxide In methanol; water at 100℃; for 0.25h; Inert atmosphere; Stage #2: 4-dimethylamino-benzaldehyde In methanol; water at 0 - 20℃; for 72h; Inert atmosphere; | 86% |
-
-
608-08-2
3-indoxyl acetate

-
-
127033-04-9
ethyl (2Z)-3-bromo-2-(hydroxyimino)propanoate

-
A
-
106040-13-5
ethyl α-(hydroxyimino)-β-(3-acetoxyindolen-3-yl)propanoate

-
-
106040-12-4
3-(ethoxycarbonyl)-4a-acetoxy-4,4a,9,9a-tetrahydro-1,2-oxazino<5,6-b>indole

| Conditions | Yield |
|---|---|
| With sodium carbonate In dichloromethane for 18h; Ambient temperature; | A 15% B 85% |


| Conditions | Yield |
|---|---|
| With sodium carbonate In methanol | 85% |
| With sodium carbonate In methanol at 20℃; for 3.5h; Inert atmosphere; | |
| With sodium carbonate In methanol |
-
-
608-08-2
3-indoxyl acetate

-
-
391-12-8
7-trifluoromethylisatin

-
-
1409933-16-9
(2’Z)-7-trifluoromethyl-indirubin

| Conditions | Yield |
|---|---|
| With sodium carbonate In methanol at 20℃; for 3.5h; Inert atmosphere; | 85% |
-
-
906660-35-3
7-bromo-1-methylindoline-2,3-dione

-
-
608-08-2
3-indoxyl acetate

-
-
906660-41-1
7-Bromo-1-methylindirubin

| Conditions | Yield |
|---|---|
| With sodium carbonate In methanol | 83% |
| With sodium carbonate In methanol at 20℃; for 3.5h; |

| Conditions | Yield |
|---|---|
| With triethylamine at 80 - 90℃; for 0.5h; | 82% |
| With dmap; triethylamine In tetrahydrofuran for 3h; Heating / reflux; | 67% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; acetic acid In water at 20℃; for 18h; Darkness; | 82% |

| Conditions | Yield |
|---|---|
| With sodium sulfate; triethylamine In tetrahydrofuran; isopropyl alcohol at 30℃; for 60h; Enzymatic reaction; | 82% |
3-Indolyl acetate Chemical Properties
Product Name: Indoxyl acetate (608-08-2)
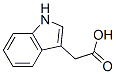
Molecular Formula: C10H9NO2
Molecular Weight: 175.18g/mol
Mol File: 608-08-2.mol
Einecs: 210-154-2
Appearance: Dark Blue Solid
Melting Point: 128-130 °C(lit.)
storage Temperature: 2-8°C
Product Categories: Indoles and derivatives ; Indole Derivatives ; Simple Indoles ; Indoles ; Intermediates .
Synonyms of Ethyl Indoxyl acetate (608-08-2) : 3-acetoxyindole ; 3-indolyl acetate ; 3-indoxyl-3-acetate ; 3-indoxyl acetate ; acetic acid 3-indolyl ester ; 1h-indol-3-yl acetate ; indol-3-yl acetate ; indoxyl acetate ; 1h-indol-3-ol 3-acetate ; 1h-indol-3-ol acetate (ester) ; 3-acetoxyindole ; acetic acid ; 3-indolyl ester ; indol-3-ol acetate (ester) (8CI) .
3-Indolyl acetate Uses
Indoxyl acetate (608-08-2) is mainly used as a plant growth-stimulating hormone and reagents.
3-Indolyl acetate Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 600mg/kg (600mg/kg) | Nippon Yakurigaku Zasshi. Japanese Journal of Pharmacology. Vol. 55, Pg. 1514, 1959. |
3-Indolyl acetate Consensus Reports
Reported in EPA TSCA Inventory.
3-Indolyl acetate Safety Profile
Moderately toxic by intraperitoneal route. When heated to decomposition it emits toxic vapors of NOx.
Safety Information of Ethyl Indoxyl acetate (608-08-2):
Hazard Codes: Xn ![]()
Risk Statements: 22-36/37/38
22: Harmful if swallowed
36: Irritating to the eyes
37: Irritating to the respiratory system
38: Irritating to the skin
Safety Statements: 26-36
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36: Wear suitable protective clothing
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View