-
Name
3-Nitro-4,5-dihydroxybenzaldehyde
- EINECS 441-810-8
- CAS No. 116313-85-0
- Article Data27
- CAS DataBase
- Density 1.667 g/cm3
- Solubility soluble in methanol
- Melting Point 147 °C
- Formula C7H5NO5
- Boiling Point 310.9 °C at 760 mmHg
- Molecular Weight 183.12
- Flash Point 141.1 °C
- Transport Information
- Appearance yellow color crystalline powder.
- Safety 26-36/37-45
- Risk Codes 25-36-43
-
Molecular Structure
- Hazard Symbols T
- Synonyms 3,4-Dihydroxy-5-nitrobenzaldehyde;Benzaldehyde, 3,4-dihydroxy-5-nitro-;BRN 3283877;
- PSA 103.35000
- LogP 1.34170
Synthetic route
-
-
6635-20-7
isonitrovanillin

-
-
116313-85-0
3,4-dihydroxy-5-nitrobenzaldehyde

| Conditions | Yield |
|---|---|
| With hydrogen bromide; acetic acid In water at 110℃; for 39h; | 100% |
| With pyridine; aluminium trichloride In chloroform for 24h; ether cleavage; Heating; | 98% |
| With hydrogen bromide; acetic acid In water for 8h; Heating; | 96% |
-
-
121-33-5
vanillin

-
-
116313-85-0
3,4-dihydroxy-5-nitrobenzaldehyde

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: diethyl ether; mixture of gaseous nitrogen oxides 2: fuming hydrochloric acid / 140 °C View Scheme | |
| Multi-step reaction with 2 steps 1: nitric acid; acetic acid / 0 - 20 °C 2: hydrogen bromide / water / 4 h / Reflux View Scheme | |
| Multi-step reaction with 2 steps 1.1: acetic acid; nitric acid / water / 4 h / 15 - 30 °C 2.1: tetrabutylammomium bromide; aluminum (III) chloride / dichloromethane / 0.5 h / 0 - 10 °C / Reflux 2.2: 25 h / 0 - 10 °C / Reflux View Scheme |

| Conditions | Yield |
|---|---|
| With acetic acid In conc. hydrobromic acid; water | 5.66 kg (80%) |
-
-
621-59-0
isovanillin

-
-
116313-85-0
3,4-dihydroxy-5-nitrobenzaldehyde

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: nitric acid / acetic acid 2: aluminum (III) chloride; pyridine / chloroform / Reflux View Scheme |
-
-
80547-69-9
3-hydroxy-4-methoxy-5-nitrobenzaldehyde

-
-
116313-85-0
3,4-dihydroxy-5-nitrobenzaldehyde

| Conditions | Yield |
|---|---|
| With pyridine; aluminum (III) chloride In chloroform Reflux; |
-
-
17722-12-2
N-(3-chlorophenyl)-2-cyanoacetamide

-
-
116313-85-0
3,4-dihydroxy-5-nitrobenzaldehyde

-
-
1150310-12-5
C16H10ClN3O5

| Conditions | Yield |
|---|---|
| With piperidine; acetic acid In toluene Knoevenagel condensation; Reflux; | 98% |
-
-
5382-38-7
2-cyano-N-(4-methoxyphenyl)acetamide

-
-
116313-85-0
3,4-dihydroxy-5-nitrobenzaldehyde

-
-
1150310-11-4
C17H13N3O6

| Conditions | Yield |
|---|---|
| With piperidine; acetic acid In toluene Knoevenagel condensation; Reflux; | 95% |
-
-
116313-85-0
3,4-dihydroxy-5-nitrobenzaldehyde

-
-
84211-30-3
3,4-dihydroxy-5-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| With sodium chlorite; sodium dihydrogenphosphate dihydrate In water; dimethyl sulfoxide at 50℃; for 16.8333h; | 94% |
| With xanthin for 20h; pH=7.4; Enzymatic reaction; |
-
-
116313-85-0
3,4-dihydroxy-5-nitrobenzaldehyde

-
-
100-39-0
benzyl bromide

-
-
312327-13-2
3-benzyloxy-4-hydroxy-5-nitro-benzaldehyde

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 140℃; for 48h; Alkylation; | 93% |
| With sodium hydride In N,N-dimethyl-formamide | 85% |
| Stage #1: 3,4-dihydroxy-5-nitrobenzaldehyde With sodium hydride In N,N-dimethyl-formamide; mineral oil at 0℃; Inert atmosphere; Stage #2: benzyl bromide In N,N-dimethyl-formamide; mineral oil at 0℃; for 1h; Inert atmosphere; Stage #3: With acetic acid In N,N-dimethyl-formamide; mineral oil at 20℃; Inert atmosphere; | 81% |
-
-
116313-85-0
3,4-dihydroxy-5-nitrobenzaldehyde

-
-
111233-69-3
2-cyano-N-[3-(2-cyanoacetylamino)propyl]acetamide

| Conditions | Yield |
|---|---|
| With 3-amino propanoic acid In ethanol for 4.5h; Heating; | 92% |
-
-
54153-19-4
2-cyano-N-m-tolylacetamide

-
-
116313-85-0
3,4-dihydroxy-5-nitrobenzaldehyde

-
-
1150310-10-3
C17H13N3O5

| Conditions | Yield |
|---|---|
| With piperidine; acetic acid In toluene Knoevenagel condensation; Reflux; | 89% |
-
-
794522-90-0
2-(pyrimidin-4-yl)acetonitrile

-
-
116313-85-0
3,4-dihydroxy-5-nitrobenzaldehyde

| Conditions | Yield |
|---|---|
| With ammonium acetate In methanol at 80℃; for 4h; | 89% |
-
-
51838-06-3
3-(N,N-diphenylamino)-3-oxopropanenitrile

-
-
116313-85-0
3,4-dihydroxy-5-nitrobenzaldehyde

-
-
1150310-09-0
C22H15N3O5

| Conditions | Yield |
|---|---|
| With piperidine; acetic acid In toluene Knoevenagel condensation; Reflux; | 88% |
-
-
59566-45-9
2-(pyrimidin-2-yl)acetonitrile

-
-
116313-85-0
3,4-dihydroxy-5-nitrobenzaldehyde

| Conditions | Yield |
|---|---|
| With ammonium acetate In methanol for 5h; Reflux; | 88% |
-
-
116313-85-0
3,4-dihydroxy-5-nitrobenzaldehyde

-
-
106-93-4
ethylene dibromide

-
-
698986-32-2
8-nitro-2,3-dihydro-benzo[1,4]dioxine-6-carbonitrilebenzonitrile

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 5h; | 87% |
-
-
3261-05-0
5-chlorobenzofuran-3(2H)-one

-
-
116313-85-0
3,4-dihydroxy-5-nitrobenzaldehyde

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water; isopropyl alcohol at 80℃; | 86% |
-
-
141-82-2
malonic acid

-
-
116313-85-0
3,4-dihydroxy-5-nitrobenzaldehyde

| Conditions | Yield |
|---|---|
| With pyridine; aniline In toluene for 2h; Reflux; | 84.6% |
| With piperidine; pyridine at 60℃; for 48h; Knoevenagel Condensation; | 40% |
-
-
110-89-4
piperidine

-
-
26391-06-0
2-cyano-N,N-diethylacetamide

-
-
116313-85-0
3,4-dihydroxy-5-nitrobenzaldehyde

-
-
1047659-01-7
entacapone; piperidine salt

| Conditions | Yield |
|---|---|
| With acetic acid In isopropyl alcohol for 3h; Product distribution / selectivity; Heating / reflux; | 79.7% |

-
-
15029-32-0
4-cyanoacetylmorpholine

-
-
116313-85-0
3,4-dihydroxy-5-nitrobenzaldehyde

-
-
1150310-16-9
C14H13N3O6

| Conditions | Yield |
|---|---|
| With piperidine; acetic acid In toluene Knoevenagel condensation; Reflux; | 78% |
| With ammonium acetate In methanol for 5h; Reflux; | 19% |
-
-
116313-85-0
3,4-dihydroxy-5-nitrobenzaldehyde

-
-
120-92-3
cyclopentanone

-
-
116313-82-7
2,5-bis(3,4-dihydroxy-5-nitrobenzylidene)cyclopentanone

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol at 5℃; | 77% |
| 4.4 g (78%) |

-
-
116313-85-0
3,4-dihydroxy-5-nitrobenzaldehyde

| Conditions | Yield |
|---|---|
| With ammonium acetate In methanol for 3h; Reflux; | 76% |
-
-
26391-06-0
2-cyano-N,N-diethylacetamide

-
-
116313-85-0
3,4-dihydroxy-5-nitrobenzaldehyde

-
-
130929-57-6
entacapone

| Conditions | Yield |
|---|---|
| Stage #1: 2-cyano-N,N-diethylacetamide; 3,4-dihydroxy-5-nitrobenzaldehyde With piperidine In 1,2-dimethoxyethane; n-heptane for 15 - 25h; Heating / reflux; Stage #2: In dichloromethane at 25 - 35℃; for 24h; Product distribution / selectivity; | 75% |
| With piperidine In isopropyl alcohol for 12 - 15h; Heating / reflux; | 75.5% |
| Stage #1: 2-cyano-N,N-diethylacetamide; 3,4-dihydroxy-5-nitrobenzaldehyde With acetic acid; diethylamine In toluene Knoevenagel Condensation; Stage #2: With hydrogen bromide In acetic acid; toluene at 20℃; | 73.8% |
-
-
26391-06-0
2-cyano-N,N-diethylacetamide

-
-
116313-85-0
3,4-dihydroxy-5-nitrobenzaldehyde

-
A
-
130929-57-6
entacapone

-
B
-
145195-63-7
(2Z)-2-cyano-3-(3,4-dihydroxy-5-nitrophenyl)-N,N-diethyl-2-propenamide

| Conditions | Yield |
|---|---|
| Stage #1: 2-cyano-N,N-diethylacetamide; 3,4-dihydroxy-5-nitrobenzaldehyde With piperidine In isopropyl alcohol for 12 - 15h; Heating / reflux; Stage #2: With acetic acid In isopropyl alcohol at 20℃; | A 75.5% B n/a |
| piperidine; methylamine hydrochloride In butan-1-ol at 82℃; for 4 - 5h; Product distribution / selectivity; | |
| piperidine; methylamine hydrochloride In i-Amyl alcohol at 89 - 90℃; for 3h; Product distribution / selectivity; |

-
-
116313-85-0
3,4-dihydroxy-5-nitrobenzaldehyde

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; sodium hydroxide In methanol; water at 60℃; for 3h; | 75% |
-
-
116313-85-0
3,4-dihydroxy-5-nitrobenzaldehyde

-
-
27349-80-0
2-(pyridazin-3-yl)acetonitrile

| Conditions | Yield |
|---|---|
| With ammonium acetate In methanol at 80℃; for 4h; | 75% |
-
-
116313-85-0
3,4-dihydroxy-5-nitrobenzaldehyde

-
-
105-53-3
diethyl malonate

| Conditions | Yield |
|---|---|
| With piperidine; pyridine at 60℃; for 7h; Knoevenagel Condensation; | 69.01% |

-
-
116313-85-0
3,4-dihydroxy-5-nitrobenzaldehyde

| Conditions | Yield |
|---|---|
| With ammonium acetate In methanol for 5h; Reflux; | 69% |
-
-
116313-85-0
3,4-dihydroxy-5-nitrobenzaldehyde

-
-
98-86-2
acetophenone

-
-
116313-76-9
3-(3,4-dihydroxy-5-nitrophenyl)-1-phenylprop-2-en-1-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol | 68% |
-
-
1071-46-1
hydrogen ethyl malonate

-
-
116313-85-0
3,4-dihydroxy-5-nitrobenzaldehyde

| Conditions | Yield |
|---|---|
| With piperidine; pyridine at 60℃; for 24h; Knoevenagel Condensation; | 67.44% |
-
-
88107-40-8
neopentyl α-cyanoacetate

-
-
116313-85-0
3,4-dihydroxy-5-nitrobenzaldehyde

| Conditions | Yield |
|---|---|
| 67% |
-
-
15029-30-8
1-cyanoacetylpiperidine

-
-
116313-85-0
3,4-dihydroxy-5-nitrobenzaldehyde

-
-
1150310-15-8
(2E)-3-(3,4-dihydroxy-5-nitrophenyl)-2-(piperidin-yl-1-carbonyl)prop-2-ennitrile

| Conditions | Yield |
|---|---|
| With piperidine; acetic acid In toluene Knoevenagel condensation; Reflux; | 67% |
| With ammonium acetate In methanol for 3h; Reflux; | 13% |
3-Nitro-4,5-dihydroxybenzaldehyde Chemical Properties
Product Name: 3-Nitro-4,5-dihydroxybenzaldehyde (CAS NO.116313-85-0)
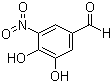
Molecular Formula: C7H5NO5
Molecular Weight: 183.12g/mol
Mol File: 116313-85-0.mol
Melting Point: 147 °C
Boiling point: 310.9 °C at 760 mmHg
Flash Point: 141.1 °C
Density: 1.667 g/cm3
Index of Refraction: 1.718
Molar Refractivity: 43.31 cm3
Molar Volume: 109.8 cm3
Surface Tension: 89.6 dyne/cm
Enthalpy of Vaporization: 57.38 kJ/mol
Vapour Pressure: 0.000319 mmHg at 25°C
XLogP3-AA: 1.1
H-Bond Donor: 2
H-Bond Acceptor: 5
Structure Descriptors of 3-Nitro-4,5-dihydroxybenzaldehyde (CAS NO.116313-85-0):
IUPAC Name: 3,4-dihydroxy-5-nitrobenzaldehyde
Canonical SMILES: C1=C(C=C(C(=C1[N+](=O)[O-])O)O)C=O
InChI: InChI=1S/C7H5NO5/c9-3-4-1-5(8(12)13)7(11)6(10)2-4/h1-3,10-11H
InChIKey: BBFJODMCHICIAA-UHFFFAOYSA-N
Product Categories: Aromatic Aldehydes & Derivatives (substituted); Benzaldehyde; pharmaceutical intermediates
3-Nitro-4,5-dihydroxybenzaldehyde Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LDLo | oral | 312mg/kg (312mg/kg) | Helvetica Chimica Acta. Vol. 72, Pg. 952, 1989. |
3-Nitro-4,5-dihydroxybenzaldehyde Safety Profile
Combustion Products: These products include toxic carbon oxides (CO,CO 2), nitrogen oxides (NOx).
SMALL FIRE: Use DRY chemical powder.
LARGE FIRE: Use water spray, fog or foam. DO NOT use water jet.
RTECS: CU5665000
3-Nitro-4,5-dihydroxybenzaldehyde Specification
3-Nitro-4,5-dihydroxybenzaldehyde , its CAS NO. is 116313-85-0, the synonyms are 3,4-Dihydroxy-5-nitrobenzaldehyde ; Benzaldehyde, 3,4-dihydroxy-5-nitro- .
Related Products
- 3-Nitro-4-(1-pyrrolidino)benzaldehyde
- 3-Nitro-4-(trifluoromethoxy)aniline
- 3-Nitro-4-(trifluoromethyl)benzoic acid
- 3-Nitro-4-(trifluoromethyl)phenol
- 3-Nitro-4,5-dihydroxybenzaldehyde
- 3-Nitro-4-chloro-4'-fluorobenzophenone
- 3-Nitro-4-fluorobenzaldehyde
- 3-Nitro-4-fluorobenzonitrile
- 3-Nitro-4-fluorobenzoyl chloride
- 3-Nitro-4-hydroxyphenylarsenous acid
- 116314-66-0
- 116316-03-1
- 1163-19-5
- 116325-74-7
- 116332-64-0
- 1163-36-6
- 116339-45-8
- 116340-67-1
- 116344-12-8
- 116344-17-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View