-
Name
3-Methylanisole
- EINECS 202-893-4
- CAS No. 100-84-5
- Article Data111
- CAS DataBase
- Density 0.941 g/cm3
- Solubility Soluble in alcohol and insoluble in water.
- Melting Point -47oC
- Formula C8H10O
- Boiling Point 172.2 °C at 760 mmHg
- Molecular Weight 122.167
- Flash Point 54.4 °C
- Transport Information UN 1993 3/PG 3
- Appearance colourless liquid
- Safety 16-23-24/25
- Risk Codes 10-22
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Anisole,m-methyl- (8CI);1-Methoxy-3-methylbenzene;3-Cresol methyl ether;3-Methoxytoluene;3-Methyl-1-methoxybenzene;Methyl m-tolylether;NSC 6255;m-Cresol methyl ether;m-Cresyl methyl ether;m-Methoxytoluene;m-Methylanisole;
- PSA 9.23000
- LogP 2.00360
Synthetic route

| Conditions | Yield |
|---|---|
| With palladium dichloride In methanol at 40℃; for 18h; Inert atmosphere; Green chemistry; chemoselective reaction; | 99% |
| With [IrCl(CO)(PPh3)2]; hydrazine hydrate; potassium hydroxide In methanol at 160℃; for 3h; Wolff-Kishner Reduction; Sealed tube; | 86% |
| With [IrCl(CO)(PPh3)2]; hydrazine hydrate; potassium hydroxide In methanol at 160℃; for 3h; Sealed tube; | 86% |
| With carbon monoxide; hydrogen; benzene unter Zusatz von Octacarbonyldikobalt und CoCO3 auf 190grad/238 at; | |
| Multi-step reaction with 3 steps 1: 78 percent / sodium iodide; sodium hydride / tetrahydrofuran / 0 - 20 °C 2: sulfuryl chloride / CH2Cl2 / -78 °C 3: lithium; 4,4'-di-tert-butylbiphenyl / tetrahydrofuran / 1 h / 0 °C View Scheme |
-
-
27060-75-9
4-bromo-3-methylanisole

-
-
100-84-5
1-methoxy-3-methyl-benzene

| Conditions | Yield |
|---|---|
| With potassium carbonate; butan-1-ol; palladium diacetate; triphenylphosphine at 100℃; for 14h; | 98% |
-
-
19924-43-7
3-methoxyphenylacetonitrile

-
-
100-84-5
1-methoxy-3-methyl-benzene

| Conditions | Yield |
|---|---|
| Stage #1: 3-methoxyphenylacetonitrile With 15-crown-5; sodium In tetrahydrofuran; hexane at 0℃; for 0.333333h; Birch Reduction; Inert atmosphere; Stage #2: With water In tetrahydrofuran; hexane at 0℃; for 1h; Birch Reduction; Inert atmosphere; chemoselective reaction; | 98% |
| Stage #1: 3-methoxyphenylacetonitrile With dibenzo-18-crown-6; sodium In tetrahydrofuran; toluene at 0℃; for 0.333333h; Inert atmosphere; Stage #2: With propan-1-ol In tetrahydrofuran; toluene at 0℃; for 1h; | 78% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-methoxy-benzaldehyde With iron(III) chloride In methanol at 20℃; for 0.05h; Stage #2: In methanol at 20℃; for 0.166667h; chemoselective reaction; | 95% |
| With borohydride exchange resin; nickel diacetate In methanol for 3h; Ambient temperature; | 93% |
| With triethylsilane; palladium dichloride In ethanol for 0.5h; Inert atmosphere; | 86.0 %Chromat. |
| With triethylsilane; palladium dichloride In ethanol at 20℃; for 0.5h; Inert atmosphere; | 86 %Chromat. |
-
-
63452-69-7
1-iodo-4-methoxy-2-methylbenzene

-
-
100-84-5
1-methoxy-3-methyl-benzene

| Conditions | Yield |
|---|---|
| With potassium carbonate; butan-1-ol; palladium diacetate; triphenylphosphine at 100℃; for 1h; | 93% |

| Conditions | Yield |
|---|---|
| With benzyltrimethylammonium chloride; sodium hydroxide In water at 70℃; Concentration; Temperature; Autoclave; | 92.2% |
-
-
155496-74-5
1-Methoxy-3-methyl-2-(2-methyl-propane-2-sulfonyl)-benzene

-
-
100-84-5
1-methoxy-3-methyl-benzene

| Conditions | Yield |
|---|---|
| With bis(acetylacetonate)nickel(II); isopropylmagnesium chloride In tetrahydrofuran at 20℃; for 20h; | 92% |

| Conditions | Yield |
|---|---|
| In methanol for 1.6h; Methylation; | 92% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 50℃; for 5h; | 91.8% |
| With sodium hydroxide |

| Conditions | Yield |
|---|---|
| With ethene; 5%-palladium/activated carbon at 130℃; under 760.051 Torr; | 88% |

| Conditions | Yield |
|---|---|
| With 20 % Pd(OH)2/C; hydrogen In methanol at 20℃; under 760.051 Torr; for 3h; | 86% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 72℃; for 24h; Inert atmosphere; | 85% |
| With sodium hydroxide In N,N-dimethyl acetamide at 25℃; Rate constant; | |
| With potassium hydroxide |

| Conditions | Yield |
|---|---|
| With 18-crown-6 ether; potassium carbonate at 150℃; for 2h; | 85% |

| Conditions | Yield |
|---|---|
| With N,N'-dimethylimidazolium-2-carboxylate In acetonitrile at 160℃; for 1.33333h; Microwave irradiation; Green chemistry; | 84% |
| With dimanganese decacarbonyl at 180℃; for 1h; Reagent/catalyst; | 81% |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene at 90℃; Inert atmosphere; |
-
-
41977-20-2
(2R,3S)-2,3-dihydroxy-1-methylcyclohexa-4,6-diene

-
-
74-88-4
methyl iodide

-
A
-
578-58-5
2-methylmethoxybenzene

-
C
-
100-84-5
1-methoxy-3-methyl-benzene

| Conditions | Yield |
|---|---|
| With potassium hydroxide In dimethyl sulfoxide at 0℃; for 1.5h; | A n/a B 80% C n/a |

-
-
1026136-94-6
2-tert-butylsulfinyl-3-methoxytoluene

-
-
100-84-5
1-methoxy-3-methyl-benzene

| Conditions | Yield |
|---|---|
| With nickel In ethanol for 8h; Reflux; | 80% |
-
-
824-98-6
m-methoxybenzyl chloride

-
A
-
36707-27-4
1,2-bis(3-methoxyphenyl)ethane

-
B
-
100-84-5
1-methoxy-3-methyl-benzene

| Conditions | Yield |
|---|---|
| With nickel In 1,2-dimethoxyethane for 6h; Ambient temperature; | A 69% B n/a |

| Conditions | Yield |
|---|---|
| Stage #1: 3-methoxyphenyl bromide With bis(tri-t-butylphosphine)palladium(0); oxygen In toluene at 20℃; for 0.0166667h; Schlenk technique; Stage #2: methyllithium In toluene at 20℃; for 0.0333333h; Schlenk technique; | 69% |
-
-
110-62-3
pentanal

-
-
1798-09-0
m-methoxyphenylacetic acid

-
A
-
1250895-32-9
1-(3-methoxyphenyl)hexan-2-ol

-
B
-
100-84-5
1-methoxy-3-methyl-benzene

| Conditions | Yield |
|---|---|
| With (4s,6s)-2,4,5,6-tetra(9H-carbazol-9-yl)isophthalonitrile; caesium carbonate In N,N-dimethyl acetamide at 20℃; for 16h; Irradiation; Inert atmosphere; | A 63% B 20 %Chromat. |

-
-
67-56-1
methanol

-
-
108-39-4
3-methyl-phenol

-
A
-
95-87-4
2,5-Dimethylphenol

-
B
-
526-75-0
2,3-Dimethylphenol

-
C
-
2416-94-6
2,3,6-trimethylphenol

-
D
-
100-84-5
1-methoxy-3-methyl-benzene

| Conditions | Yield |
|---|---|
| silica gel at 350℃; under 61504.9 Torr; Product distribution; Further Variations:; Catalysts; Pressures; Temperatures; | A 46% B 9% C 38% D n/a |

-
-
863727-67-7
2-methoxy-6-methylphenyl trifluoromethanesulfonate

-
-
100-84-5
1-methoxy-3-methyl-benzene

| Conditions | Yield |
|---|---|
| With formic acid; tributyl-amine; 1,3-bis-(diphenylphosphino)propane; bis-triphenylphosphine-palladium(II) chloride In N,N-dimethyl-formamide at 80℃; for 8h; | 46% |
-
-
194018-68-3
vanillin triflate

-
A
-
6971-51-3
3-methoxybenzyl alcohol

-
B
-
100-84-5
1-methoxy-3-methyl-benzene

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In ethyl acetate at 20℃; under 760.051 Torr; for 40h; | A 30% B 42% |
-
-
67-56-1
methanol

-
-
108-95-2
phenol

-
A
-
104-93-8
4-Methylanisole

-
B
-
578-58-5
2-methylmethoxybenzene

-
C
-
1004-66-6
2-methoxy-1,3-dimethylbenzene

-
D
-
6738-23-4
2,4-dimethylanisole

-
E
-
100-66-3
methoxybenzene

-
F
-
100-84-5
1-methoxy-3-methyl-benzene

| Conditions | Yield |
|---|---|
| APTi-II-770 at 349.9℃; Product distribution; alkylation; other catalysts, other temperatures; also cresols, further products; | A n/a B 1.8% C n/a D n/a E 19% F n/a |

-
-
25649-41-6
4,4',4''-((1,3,5-triazine-2,4,6-triyl)tris(oxy))tris(3-methoxybenzaldehyde)

-
-
100-84-5
1-methoxy-3-methyl-benzene

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In ethyl acetate at 20℃; for 24h; | 14% |

| Conditions | Yield |
|---|---|
| With water; tris-(dibenzylideneacetone)dipalladium(0); trifuran-2-yl-phosphane In tetrahydrofuran Heating; | 3% |

| Conditions | Yield |
|---|---|
| With diethyl ether; magnesium anschliessend Behandeln mit Dimethylsulfat, zuletzt auf dem Dampfbad; |
-
-
60-29-7
diethyl ether

-
-
38197-43-2
2-bromo-3-methylanisole

-
-
3585-33-9
lithium dimethylamide

-
B
-
100-84-5
1-methoxy-3-methyl-benzene

-
-
5456-94-0
2,4-dibromo-5-methylanisole

-
A
-
27060-75-9
4-bromo-3-methylanisole

-
B
-
100-84-5
1-methoxy-3-methyl-benzene

| Conditions | Yield |
|---|---|
| With diethyl ether; magnesium anschliessende Hydrolyse; |

| Conditions | Yield |
|---|---|
| at 400 - 420℃; durch Ueberleiten ueber ThO2; |
-
-
100-84-5
1-methoxy-3-methyl-benzene

-
-
27060-75-9
4-bromo-3-methylanisole

| Conditions | Yield |
|---|---|
| With hydrogenchloride; N-Bromosuccinimide; water In acetone at 20℃; for 0.0833333h; Inert atmosphere; | 100% |
| With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione; methyl 2-n-pentyl-1H-indole-3-carboxylate In n-heptane at 23℃; for 16h; Darkness; Green chemistry; regioselective reaction; | 99% |
| With N-Bromosuccinimide; 2,4,6-trimethylaniline In dichloromethane at -40℃; for 2h; Inert atmosphere; regioselective reaction; | 98% |
-
-
100-84-5
1-methoxy-3-methyl-benzene

-
-
63452-69-7
1-iodo-4-methoxy-2-methylbenzene

| Conditions | Yield |
|---|---|
| With iodine; n-butyltriphenylphosphonium peroxodisulfate In acetonitrile for 0.5h; Heating; | 100% |
| With N,N,N-trimethylbenzenemethanaminium dichloroiodate; zinc(II) chloride In acetic acid for 0.5h; Ambient temperature; | 94% |
| With N-iodo-succinimide; 2,4,6-trifluoroaniline In dichloromethane at 20℃; for 24h; Inert atmosphere; regioselective reaction; | 94% |
-
-
100-84-5
1-methoxy-3-methyl-benzene

| Conditions | Yield |
|---|---|
| Stage #1: 1-methoxy-3-methyl-benzene With potassium tert-butylate at -35℃; for 0.333333h; Stage #2: With n-butyllithium In hexane at -35 - 20℃; for 12h; | 100% |
-
-
100-84-5
1-methoxy-3-methyl-benzene

-
-
19614-12-1
2-bromo-5-methoxybenzyl bromide

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In dichloromethane for 4h; Heating; IR lamp; | 99% |
| With N-Bromosuccinimide In dichloromethane for 4h; Bromination; Heating; irradiation; | 88% |
| With N-Bromosuccinimide Photolysis; | 88% |
-
-
13274-43-6
4-methyl-1,2,4-triazoline-3,5-dione

-
-
100-84-5
1-methoxy-3-methyl-benzene

-
-
1268396-59-3
1-(4-methoxy-2-methylbenzene)-4-methyl-1,2,4-triazoline-3,5-dione

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In chloroform at 62℃; for 0.166667h; regioselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With tetrabutylammonium acetate for 4.5h; Electrolysis; Inert atmosphere; Sealed tube; regioselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; | 98% |
-
-
100-84-5
1-methoxy-3-methyl-benzene

-
A
-
1193-18-6
3-methylcyclohexen-2-one

-
B
-
591-24-2, 625-96-7
3-Methylcyclohexanone

| Conditions | Yield |
|---|---|
| With N,N,N,N,N,N-hexamethylphosphoric triamide; lithium In tetrahydrofuran; ethanol at -30℃; for 2h; | A 97% B 3% |
-
-
100-84-5
1-methoxy-3-methyl-benzene

-
-
13334-71-9
1-chloro-4-methoxy-2-methylbenzene

| Conditions | Yield |
|---|---|
| With N-chloro-succinimide; 2,4,6-trimethylaniline In dichloromethane at 20℃; Inert atmosphere; regioselective reaction; | 96% |
| With aluminum oxide; sodium chlorite; (salen)Mn(III) In dichloromethane at 20℃; for 0.5h; | 87% |
| With Oxone; potassium chloride In water; acetonitrile at 20℃; | 76% |
-
-
100-84-5
1-methoxy-3-methyl-benzene

-
-
117934-78-8
2,4-diiodo-5-methyl-methoxybenzene

| Conditions | Yield |
|---|---|
| With N,N,N-trimethylbenzenemethanaminium dichloroiodate; zinc(II) chloride In acetic acid for 24h; Ambient temperature; | 96% |
| With iodine; mercury(II) oxide In dichloromethane for 20h; Ambient temperature; | 80% |
| With sulfuric acid; iodine; periodic acid In water; acetic acid |
-
-
100-84-5
1-methoxy-3-methyl-benzene

-
-
82101-67-5
1-deuteriomethyl-3-methoxybenzene

| Conditions | Yield |
|---|---|
| Stage #1: 1-methoxy-3-methyl-benzene With 2,2,6,6-tetramethyl-piperidine; n-butyllithium; potassium tert-butylate In tetrahydrofuran; hexane at -78℃; Inert atmosphere; Stage #2: With d(4)-methanol In tetrahydrofuran; hexane Inert atmosphere; regioselective reaction; | 96% |
-
-
100-84-5
1-methoxy-3-methyl-benzene

-
-
24318-46-5
3-methoxybenzyl 3-methoxybenzoate

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; tetra-(n-butyl)ammonium iodide In water at 80℃; for 6h; | 96% |

| Conditions | Yield |
|---|---|
| With N,N,N,N,N,N-hexamethylphosphoric triamide; sodium hydride; N-methylaniline In diethyl ether; xylene at 120℃; for 6.5h; | 95% |
| With trimethylsilyl iodide at 105 - 114℃; for 0.25h; Microwave irradiation; Inert atmosphere; | 92% |
| With 1,3-dimethyl-2-imidazolidinone; sodium hexamethyldisilazane In tetrahydrofuran at 185℃; for 12h; further reagent: LDA; | 87% |

| Conditions | Yield |
|---|---|
| In methanol; water at 20℃; for 2h; | 95% |
| With trifluoroacetic acid |

-
-
100-84-5
1-methoxy-3-methyl-benzene

-
-
952514-33-9
[(2,6-bis(diphenylphosphinomethyl)pyridine)Pt(C2H4C6H3(OMe)Me)]SbF6

-
-
952514-35-1
[(2,6-bis(diphenylphosphinomethyl)pyridine)Pt(C2H4C6H3(OMe)Me)]SbF6

| Conditions | Yield |
|---|---|
| In dichloromethane; water aryl compd. (1.5 equiv.) and H2O were added to soln. of Pt complex in CH2Cl2; mixt. was stirred for 2 h; dried (Na2SO4); filtered; concd. (vac.); Et2O added dropwise; filtered; dried (vac.); elem. anal.; | A 95% B n/a |


-
-
100-84-5
1-methoxy-3-methyl-benzene

| Conditions | Yield |
|---|---|
| With iron(III) chloride; silver hexafluoroantimonate In 1,2-dichloro-ethane at 100℃; for 16h; Inert atmosphere; Sealed tube; | 95% |
-
-
507-20-0
tertiary butyl chloride

-
-
100-84-5
1-methoxy-3-methyl-benzene

-
-
88-40-4
2-tert-butyl-5-methylanisole

| Conditions | Yield |
|---|---|
| rhenium(I) pentacarbonyl bromide In 1,2-dichloro-ethane at 84℃; for 5h; Product distribution; | 93% |
| Stage #1: 1-methoxy-3-methyl-benzene With aluminum (III) chloride In dichloromethane at 0 - 5℃; for 1h; Stage #2: tertiary butyl chloride In dichloromethane at 0 - 5℃; for 5h; Temperature; | 87.46% |
| With phosphoric acid at 55 - 60℃; | |
| With aluminium trichloride | |
| With aluminium trichloride |

| Conditions | Yield |
|---|---|
| With [2,2]bipyridinyl; ammonia; dihydrogen peroxide; oxygen In N,N-dimethyl-formamide at 80℃; under 1500.15 - 3000.3 Torr; for 6h; Autoclave; | 93% |
| With tert.-butylnitrite; N-hydroxyphthalimide; palladium diacetate In acetonitrile at 70℃; for 24h; Inert atmosphere; Sealed tube; | 81% |
| With air; ammonia; vanadium-titanium oxide at 383.9℃; for 0.000333333h; | 16.1 |

| Conditions | Yield |
|---|---|
| Stage #1: dicyanozinc; 1-methoxy-3-methyl-benzene With hydrogenchloride In 1,1,2,2-tetrachloroethane at 17℃; Stage #2: With aluminium trichloride In 1,1,2,2-tetrachloroethane at 55℃; for 3h; | 93% |
-
-
140-10-3
(E)-3-phenylacrylic acid

-
-
100-84-5
1-methoxy-3-methyl-benzene

-
A
-
131866-19-8
3-(4-hydroxy-2-methyl-phenyl)-3-phenyl-propionic acid

-
B
-
109089-78-3
3-(4-methoxy-2-methyl-phenyl)-3-phenyl-propionic acid

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid at 125℃; for 3h; | A 5% B 93% |

-
-
69180-49-0
1-diacetoxy-2-methoxyphenyl-λ3-iodane

-
-
76-05-1
trifluoroacetic acid

-
-
100-84-5
1-methoxy-3-methyl-benzene

| Conditions | Yield |
|---|---|
| In dichloromethane at -30 - 20℃; | 93% |

-
-
100-84-5
1-methoxy-3-methyl-benzene

-
-
57356-79-3
4-hydroxy-3-methyl-cyclohex-2-en-1-one

| Conditions | Yield |
|---|---|
| Stage #1: 1-methoxy-3-methyl-benzene With ammonia; lithium; tert-butyl alcohol In diethyl ether at -78℃; Birch reduction; Inert atmosphere; Stage #2: With oxalic acid In methanol; water at 20℃; for 1h; Stage #3: With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 20℃; for 3h; Inert atmosphere; | 93% |
-
-
4294-57-9
para-methylphenylmagnesium bromide

-
-
100-84-5
1-methoxy-3-methyl-benzene

-
-
7383-90-6
3,4'-dimethylbiphenyl

| Conditions | Yield |
|---|---|
| With bis(1,5-cyclooctadiene)nickel (0); C23H28N4 In tetrahydrofuran; m-xylene at 60℃; for 4h; Kumada Cross-Coupling; Inert atmosphere; Sealed tube; | 93% |
| With (IPr)Ni(π-allyl)Cl In tetrahydrofuran at 60℃; for 12h; Kumada Cross-Coupling; Inert atmosphere; | 81% |
-
-
100-84-5
1-methoxy-3-methyl-benzene

-
-
13697-84-2
1-methoxy-5-methyl-cyclohexa-1,4-diene

| Conditions | Yield |
|---|---|
| With lithium In ethanol; ammonia at -78℃; for 0.5h; | 92% |
| With ammonia; sodium In diethyl ether at -78 - -35℃; for 2h; | 82% |
| With ammonia; sodium In diethyl ether; tert-butyl alcohol at -78 - -35℃; for 2h; | 82% |
3-methylanisole Chemical Properties
The MF of 3-methylanisole(100-84-5): C8H10O
The MW of 3-methylanisole(100-84-5): 122.16
EINECS: 202-893-4
bp: 175-176 °C(lit.)
density: 0.969 g/mL at 25 °C(lit.)
refractive index: n20/D 1.513(lit.)
Fp: 130 °F
storage temp.: Flammables area
Appearance: Clear very slight yellow liquid.
Solubility in water: Insoluble
Stability: Stable. Flammable. Incompatible with strong oxidizing agents.
Categories: Aromatic Hydrocarbons (substituted) & Derivatives;Aromatic Ethers;AnisoleSeries;Anisoles, Alkyloxy Compounds & Phenylacetates;Ethers;Organic Building Blocks;Oxygen Compounds
Synonyms: 1-METHOXY-3-METHYLBENZENE;AMBROL;3-METHOXYTOLUENE;3-METHYLANISOLE;METHYL M-TOLYL ETHER;METHYL-M-CRESOL;M-CRESOL METHYL ETHER;M-CRESYL METHYL ETHER
The Structure of 3-methylanisole(100-84-5):
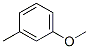
3-methylanisole Uses
3-methylanisole Toxicity Data With Reference
"Summary Tables of Biological Tests," National Research Council Chemical-Biological Coordination Center. Vol. 6, Pg. 214, 1954.
3-methylanisole Safety Profile
 Xn
Xn Risk Statements: 10-22
10: Flammable
22: Harmful if swallowed
Safety Statements: 16-23-24/25
16: Keep away from sources of ignition - No smoking
23: Do not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer)
24/25: Avoid contact with skin and eyes
RIDADR: UN 1993 3/PG 3
WGK Germany: 3
RTECS: BZ8778000
HazardClass: 3
PackingGroup: III
3-methylanisole Specification
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid immediately. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of soap and water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Eyes:
Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
2. Handling and Storage
Storage:
Keep away from heat, sparks, and flame. Keep away from sources of ignition. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Flammables-area.
Handling:
Wash thoroughly after handling. Use only in a well ventilated area. Ground and bond containers when transferring material. Use spark-proof tools and explosion proof equipment. Avoid contact with eyes, skin, and clothing. Empty containers retain product residue, (liquid and/or vapor), and can be dangerous. Keep container tightly closed. Avoid contact with heat, sparks and flame. Avoid ingestion and inhalation. Do not pressurize, cut, weld, braze, solder, drill, grind, or expose empty containers to heat, sparks or open flames.
Related Products
- 3-methylanisole
- 3-Methylanisole
- 100846-24-0
- 100848-70-2
- 1008506-24-8
- 100853-39-2
- 100853-58-5
- 100-85-6
- 100858-27-3
- 100858-32-0
- 100858-33-1
- 100859-32-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View