 This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
-
Name
4,4-Piperidinediol hydrochloride
- EINECS 254-779-9
- CAS No. 40064-34-4
- Article Data2
- CAS DataBase
- Density
- Solubility Soluble in water.
- Melting Point 97-99 °C
- Formula C5H11NO2.HCl
- Boiling Point 256.8 °C at 760 mmHg
- Molecular Weight 153.609
- Flash Point 147.9 °C
- Transport Information
- Appearance white crystalline solid
- Safety 26-36-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 2,3,5,6-tetrahydropyridin-4-one;Piperonylamine;Piperidone HCL;4,4-Dihydroxypiperidine hydrochloride;4-Pieridone monohydrochloride;
- PSA 52.49000
- LogP 0.18150
Synthetic route

-
-
40064-34-4
4-piperidone monohydrochloride monohydrate

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water |
-
-
40064-34-4
4-piperidone monohydrochloride monohydrate

-
-
6092-80-4
phenoxyamine hydrochloride

-
-
49540-52-5
1,2,3,4-tetrahydro[1]benzofuro[3,2-c]pyridine hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In isopropyl alcohol | 100% |
-
-
40064-34-4
4-piperidone monohydrochloride monohydrate

-
-
149-73-5
trimethyl orthoformate

-
-
77542-16-6
4,4-dimethoxypiperidine monohydrochloride

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In methanol at 20℃; for 22h; | 100% |
-
-
40064-34-4
4-piperidone monohydrochloride monohydrate

-
-
76-83-5
trityl chloride

-
-
112257-60-0
1-triphenylmethyl-4-piperidone

| Conditions | Yield |
|---|---|
| With triethylamine In N-methyl-acetamide; water | 98.3% |
| With triethylamine In N,N-dimethyl-formamide at 60℃; for 5h; | 70.2% |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
40064-34-4
4-piperidone monohydrochloride monohydrate

-
-
79099-07-3
N-tert-butyloxycarbonylpiperidin-4-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In 1,4-dioxane; water for 12h; Cooling with ice; | 94% |
| With sodium hydroxide In 1,4-dioxane | 92.5% |
| With triethylamine In dichloromethane; ethyl acetate | 86% |
-
-
40064-34-4
4-piperidone monohydrochloride monohydrate

-
-
497-19-8
sodium carbonate

-
-
1514-96-1
4-chloro-2-(trifluoromethyl)pyrimidine

| Conditions | Yield |
|---|---|
| In dichloromethane | 92% |

-
-
61380-07-2
2-(thiophen-2-yl)ethyl methanesulfonate

-
-
40064-34-4
4-piperidone monohydrochloride monohydrate

-
-
92065-14-0
N-[2-(2-thienyl)ethyl]-4-piperidinone

| Conditions | Yield |
|---|---|
| Stage #1: 4-piperidone monohydrochloride monohydrate With N-benzyl-N,N,N-triethylammonium chloride; potassium carbonate In acetonitrile at 60℃; for 1h; Stage #2: 2-(thiophen-2-yl)ethyl methanesulfonate In acetonitrile at 80℃; for 24h; Reagent/catalyst; | 90% |
-
-
51417-51-7
7-bromo-1H-indole

-
-
40064-34-4
4-piperidone monohydrochloride monohydrate

-
-
312631-09-7
7-bromo-3-piperidin-4-yl-1H-indole

| Conditions | Yield |
|---|---|
| 89% |
-
-
2219-51-4
[1,1,2,2-(2)H4]ethane-1,2-diol

-
-
40064-34-4
4-piperidone monohydrochloride monohydrate

-
-
84376-17-0
2,2,3,3-tetradeutero-1,4-dioxa-8-azaspiro<4,5>decane

| Conditions | Yield |
|---|---|
| In benzene for 48h; Heating; | 88% |

-
-
40064-34-4
4-piperidone monohydrochloride monohydrate

| Conditions | Yield |
|---|---|
| Stage #1: C16H15FO2 With 4-methyl-morpholine; 1-[(1-(cyano-2-ethoxy-2-oxoethylidenaminooxy)dimethylamino-morpholino)]-uronium hexafluorophosphate In N,N-dimethyl-formamide for 0.166667h; Cooling with ice; Stage #2: 4-piperidone monohydrochloride monohydrate In N,N-dimethyl-formamide at 0 - 20℃; | 88% |
-
-
5825-45-6
1-amino-1,2,3,4-tetrahydroquinoline

-
-
40064-34-4
4-piperidone monohydrochloride monohydrate

| Conditions | Yield |
|---|---|
| With hydrogenchloride | 85% |
-
-
5825-45-6
1-amino-1,2,3,4-tetrahydroquinoline

-
-
40064-34-4
4-piperidone monohydrochloride monohydrate

| Conditions | Yield |
|---|---|
| With hydrogenchloride In ethanol for 3h; Reflux; | 85% |
-
-
20667-05-4
2-methyl-1,2-pentanediol

-
-
40064-34-4
4-piperidone monohydrochloride monohydrate

| Conditions | Yield |
|---|---|
| With p-toluenesulfonic acid monohydrate In toluene | 84% |
-
-
448-19-1
4-fluoro-2-methoxy-1-nitrobenzene

-
-
40064-34-4
4-piperidone monohydrochloride monohydrate

-
-
761440-64-6
1-(3-methoxy-4 nitrophenyl)piperidin-4-one

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In acetonitrile at 80℃; Inert atmosphere; | 84% |
-
-
1006-94-6
5-methoxylindole

-
-
40064-34-4
4-piperidone monohydrochloride monohydrate

-
-
52157-82-1
4-(5-methoxy-1H-indol-3-yl)piperidine

| Conditions | Yield |
|---|---|
| 83% |
-
-
40064-34-4
4-piperidone monohydrochloride monohydrate

-
-
41840-28-2
S-tert-butoxycarbonyl-4,6-dimethyl-2-mercaptopyrimidine

-
-
79099-07-3
N-tert-butyloxycarbonylpiperidin-4-one

| Conditions | Yield |
|---|---|
| With triethylamine In 1,4-dioxane; water for 6h; Ambient temperature; | 81.3% |

-
-
40064-34-4
4-piperidone monohydrochloride monohydrate

| Conditions | Yield |
|---|---|
| With acetic anhydride; potassium carbonate In acetonitrile at 20℃; for 5h; | 81.2% |
-
-
19335-11-6
1H-indazol-5-ylamine

-
-
40064-34-4
4-piperidone monohydrochloride monohydrate

-
-
144-55-8
sodium hydrogencarbonate

-
-
611-19-8
1-chloro-2-(chloromethyl)benzene

| Conditions | Yield |
|---|---|
| With potassium carbonate; acetic acid In methanol; acetonitrile | 80% |

-
-
19335-11-6
1H-indazol-5-ylamine

-
-
40064-34-4
4-piperidone monohydrochloride monohydrate

-
-
144-55-8
sodium hydrogencarbonate

-
-
352-11-4
1-chloromethyl-4-fluorobenzene

| Conditions | Yield |
|---|---|
| With potassium carbonate; acetic acid In methanol; acetonitrile | 79% |

-
-
19335-11-6
1H-indazol-5-ylamine

-
-
40064-34-4
4-piperidone monohydrochloride monohydrate

-
-
144-55-8
sodium hydrogencarbonate

-
-
620-20-2
1-Chloro-3-chloromethyl-benzene

| Conditions | Yield |
|---|---|
| With potassium carbonate In methanol; acetonitrile | 79% |

-
-
19335-11-6
1H-indazol-5-ylamine

-
-
40064-34-4
4-piperidone monohydrochloride monohydrate

-
-
144-55-8
sodium hydrogencarbonate

-
-
104-83-6
1-Chloro-4-(chloromethyl)benzene

| Conditions | Yield |
|---|---|
| With potassium carbonate; acetic acid In methanol; acetonitrile | 79% |

-
-
23468-31-7
6-chloropiperonyl chloride

-
-
19335-11-6
1H-indazol-5-ylamine

-
-
40064-34-4
4-piperidone monohydrochloride monohydrate

-
-
144-55-8
sodium hydrogencarbonate

| Conditions | Yield |
|---|---|
| With potassium carbonate; acetic acid In methanol; acetonitrile | 78% |

-
-
40064-34-4
4-piperidone monohydrochloride monohydrate

-
-
87-42-3
6-chloro-7H-purine

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 50℃; for 2h; | 77% |
-
-
19335-11-6
1H-indazol-5-ylamine

-
-
40064-34-4
4-piperidone monohydrochloride monohydrate

-
-
144-55-8
sodium hydrogencarbonate

-
-
104-82-5
4-Methylbenzyl chloride

| Conditions | Yield |
|---|---|
| With potassium carbonate; acetic acid In methanol; acetonitrile | 76% |

-
-
50709-33-6
2-bromophenylhydrazine hydrochloride

-
-
40064-34-4
4-piperidone monohydrochloride monohydrate

-
-
1059630-11-3
6-bromo-2,3,4,5-tetrahydro-1H-pyrido[4,3-b]indole hydrochloric acid salt

| Conditions | Yield |
|---|---|
| With hydrogenchloride In ethanol for 6h; Reflux; Inert atmosphere; | 76% |
-
-
40064-34-4
4-piperidone monohydrochloride monohydrate

-
-
5451-40-1
2,6-dichloro-7H-purine

| Conditions | Yield |
|---|---|
| With triethylamine In isopropyl alcohol at 60℃; for 2h; | 76% |
-
-
19335-11-6
1H-indazol-5-ylamine

-
-
40064-34-4
4-piperidone monohydrochloride monohydrate

-
-
144-55-8
sodium hydrogencarbonate

-
-
836-42-0
4-benzyloxybenzyl chloride

| Conditions | Yield |
|---|---|
| With potassium carbonate; acetic acid In methanol; acetonitrile | 75% |

-
-
19335-11-6
1H-indazol-5-ylamine

-
-
40064-34-4
4-piperidone monohydrochloride monohydrate

-
-
144-55-8
sodium hydrogencarbonate

-
-
824-94-2
p-methoxybenzyl chloride

| Conditions | Yield |
|---|---|
| With potassium carbonate; acetic acid In methanol; acetonitrile | 74% |

-
-
948-65-2
2-phenyl-indole

-
-
40064-34-4
4-piperidone monohydrochloride monohydrate

-
-
221109-25-7
2-phenyl-3-(1,2,3,6-tetrahydropyridin-4-yl)-1H-indole

| Conditions | Yield |
|---|---|
| With phosphoric acid In acetic acid; ethyl acetate | 73% |
-
-
92259-86-4
1-amino-2,3-dihydroindole hydrochloride

-
-
40064-34-4
4-piperidone monohydrochloride monohydrate

-
-
313666-86-3
4,5,7,8,9,10-hexahydropyrido[4,3-b]pyrrolo[3,2,1-hi]indole

| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 4h; Reflux; | 73% |
-
-
40064-34-4
4-piperidone monohydrochloride monohydrate

-
-
72482-64-5
2,4-difluorobenzoyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane | 71% |
4,4-Piperidinediol hydrochloride Chemical Properties
MF: C5H12ClNO2
MW: 153.61
EINECS: 254-779-9
4-PIPERIDONE HYDRATE HYDROCHLORIDE(40064-34-4),its melting point is about 97-99 °C;appearance is White Crystalline Solid.
Following is the molecular structure of 4-PIPERIDONE HYDRATE HYDROCHLORIDE(40064-34-4):
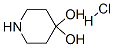
4,4-Piperidinediol hydrochloride Uses
4-PIPERIDONE HYDRATE HYDROCHLORIDE can be used in Synthesis pharmaceutical intermediates and API etc.
4,4-Piperidinediol hydrochloride Safety Profile
Safty informations about 4-PIPERIDONE HYDRATE HYDROCHLORIDE(40064-34-4):
Hazard Codes : Xi (Irritant)
Risk Statements :
R36/37/38 (Irritating to eyes, respiratory system and skin )
Safety Statements :
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
S36:Wear suitable protective clothing
S37/39 :Wear suitable gloves and eye/face protection
WGK Germany :3
4,4-Piperidinediol hydrochloride Specification
The 4-Piperidone hydrate hydrochloride, with the CAS registry number 40064-34-4, is also known as 4-Pieridone hydrochloride monohydrate. It belongs to the product categories of Nitrogen cyclic compounds; Amines and Anilines; Carbonyl Compounds; Miscellaneous; Piperidine; Miscellaneous Reagents; Building Blocks; Heterocyclic Building Blocks; Piperidones. Its EINECS registry number is 254-779-9. This chemical's molecular formula is C5H11NO2.HCl and molecular weight is 153.61. Its IUPAC name is called piperidin-4-one hydrate hydrochloride. In addition, it is white crystalline solid which should be sealed in cool, dry place.
Physical properties of 4-Piperidone hydrate hydrochloride: (1)ACD/LogP: -0.74; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -3.68; (4)ACD/LogD (pH 7.4): -2.29; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 1; (8)ACD/KOC (pH 7.4): 1; (9)#H bond acceptors: 2; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 0; (12)Flash Point: 84.6 °C; (13)Melting Point: 97-99 °C; (14)Enthalpy of Vaporization: 41.14 kJ/mol; (15)Boiling Point: 175.1 °C at 760 mmHg; (16)Vapour Pressure: 1.17 mmHg at 25°C.
Uses of 4-Piperidone hydrate hydrochloride: it can be used to produce 3-(1,2,3,6-tetrahydro-pyridin-4-yl)-indole. This reaction will need reagent 2N KOH and solvent methanol with reaction time of 8.5 hours. The yield is about 55%.
.png)
When you are using this chemical, please be cautious about it as the following:
This chemical may cause inflammation to the skin or other mucous membranes. It is irritating to eyes, respiratory system and skin. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. Whenever you will contact it, please wear suitable protective clothing, gloves and eye/face protection.
You can still convert the following datas into molecular structure:
(1)Canonical SMILES: C1CNCCC1=O.O.Cl
(2)InChI: InChI=1S/C5H9NO.ClH.H2O/c7-5-1-3-6-4-2-5;;/h6H,1-4H2;1H;1H2
(3)InChIKey: SXWRTZOXMUOJER-UHFFFAOYSA-N
Related Products
- 4-06-00-02342 (Beilstein Handbook Reference)
- 4,10-Ace-1,2-benzanthracene
- 4,10-Dioxatricyclo[5.2.1.0(2,6)]decan-8-en-3-one
- 4-(1,1,2,2-Tetrafluoroethoxy)benzoicacid
- 4-(1,1,2,2-Tetrafluoroethoxy)chlorobenzene
- 4-(1,1,2,2-Tetrafluoroethoxy)nitrobenzene
- 4-(1,1,2,2-Tetrafluoroethoxy)toluene
- 4-(1,1-Difluoropropan-2-yl)benzene-1-sulfonyl chloride
- 4-(1,1-Dioxothiazolidin-2-yl)benzoate
- 4′-(1,2,3,4-TETRAHYDRO-4-(4-HYDROXY-2-OXO-2H-1-BENZOPYRAN-3-YL)-2-NAPHTHALENYL)(1,1′-BIPHENYL)-4-CARBONITRILE, cis-
- 40064-68-4
- 400-65-7
- 40067-80-9
- 40067-82-1
- 400-67-9
- 400-70-4
- 40070-59-5
- 400716-17-8
- 400-72-6
- 400727-57-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View